The effect of living environment on developmental disorders in cold regions
doi: 10.2478/fzm-2023-0004
-
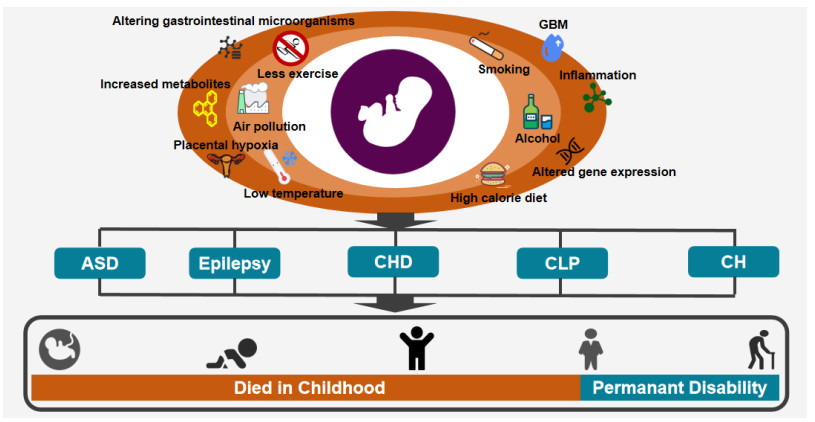
Abstract: Developmental disorders (DDs) are a kind of chronic maladies, which can cause serious irreversible detriment to children's physical and mental health. It is predominantly regulated by the interaction of environment and heredity. Cold regions are mainly located in the high latitudes of China. Their living environment is characterized by frequent cold wave, huge temperature difference, severe air pollution, high calorie diet, less exercise, smoking, drinking, etc. In recent years, substantial advances have been made in studies of the correlation between the living environment features in cold regions and the DDs. Accordingly, this article reviews the impact of the peculiar living environment of cold regions on DDs, with a view to provide fresh prevention strategies for reducing the morbidity of DDs in China cold regions by ameliorating living environment.
-
Key words:
- developmental disorders /
- cold region, low temperature /
- air pollution /
- lifestyle
-
Table 1. Representative studies on proving high prevalence of DDs in cold regions
DDs References Country Number in study Purposes Conclusions ASD Sun et al.[23] Jilin, China
Shenzhen, China20 000 children To determin the prevalence of ASD in children in Jilin and Shenzhen The prevalence of ASD in school-age children in Jilin and Shenzhen was 108 per 10 000 and 42 per 10 000, respectively Epilepsy Zheng et al.[34] Hainan Province of China 16 676 participants To assess the epidemiological characteristics of epilepsy in the tropical rural areas of Hainan Province of China The prevalence of epilepsy in the tropical rural areas of Hainan Province of China was 0.24 per 1 000 Epilepsy Syvertsen et al.[35] Nordic countries The article included 38 original articles. To conduct an evidence-based assessment of the prevalence of epilepsy in Nordic countries The highest prevalence of epilepsy in Nordic countries was 7.6 per 1 000 CHD Liu et al.[43] Xinjiang, China 14 530 school-age children To evaluate the prevalence of CHD in Xinjiang, China The prevalence of CHD in Xinjiang was 16.5 per 1 000 CHD Han et al.[44] Southwest China 244 023 children To collect epidemiological data of CHD in school children in southwest China The prevalence of CHD in children in southwest China was 6.9 per 1 000 CLP Zhu et al.[50] Guangdong Province, China 7 134 693 infants To explore the temporal and spatial distribution of CLP The prevalence of CLP in infants in Guangdong province was 7.55 per 10 000 CLP Alonso et al.[51] Colombia 15 225 participants To estimate the prevalence of CLP in Columbia from 2009 to 2017 The prevalence of CLP in Columbia was 3.27 per 10 000 CH Tamber et al.[59] - Data from the WHO-affiliated ICBDSR To review the epidemiology of CH in infants The prevalence of CH in India was 10.4 per 10 000 CH Liu et al.[60] China 176 223 livebirths To evaluate the epidemiology of CH in China The prevalence of CH in China was 20.3 per 10 000 DDs, developmental disorders; ASD, autism spectrum disorder; CHD, congenital heart disease; CLP, cleft lip and palate; CH, congenital hydrocephalus; WHO, World Health Organization; ICBDSR, International Clearinghouse for Birth Defects Surveillance and Research. -
[1] Corsello G, Giuffrè M. Congenital malformations. J Matern Fetal Neonatal Med, 2012; 25 Suppl 1: 25-29. [2] Baldacci S, Gorini F, Santoro M, et al. Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Esposizione ambientale e individuale e rischio di anomalie congenite: una rassegna delle evidenze epidemiologiche recenti. Epidemiol Prev, 2018; 42(3-4 Suppl 1): 1-34. [3] Groisman B, Bermejo-Sánchez E, Romitti P A, et al. Join World Birth Defects Day. Pediatr Res, 2019; 86(1): 3-4. doi: 10.1038/s41390-019-0392-x [4] Global PaedSurg Research Collaboration. Mortality from gastrointestinal congenital anomalies at 264 hospitals in 74 low-income, middle-income, and high-income countries: a multicentre, international, prospective cohort study. Lancet, 2021; 398(10297): 325-339. doi: 10.1016/S0140-6736(21)00767-4 [5] Dai L, Zhu J, Liang J, et al. Birth defects surveillance in China. World J Pediatr, 2011; 7(4): 302-310. doi: 10.1007/s12519-011-0326-0 [6] Agopian A J, Evans J A, Lupo P J. Analytic methods for evaluating patterns of multiple congenital anomalies in birth defect registries. Birth Defects Res, 2018; 110(1): 5-11. doi: 10.1002/bdr2.1115 [7] Paudel P, Sunny A K, Gurung R, et al. Burden and consequence of birth defects in Nepal-evidence from prospective cohort study. BMC Pediatr, 2021; 21(1): 81. doi: 10.1186/s12887-021-02525-2 [8] Zhou Y, Mao X, Zhou H, et al. Birth defects data from population-based birth defects surveillance system in a district of southern Jiangsu, China, 2014-2018. Front Public Health, 2020; 8: 378. doi: 10.3389/fpubh.2020.00378 [9] Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob Health, 2018; 6(10): e1100-e1121. doi: 10.1016/S2214-109X(18)30309-7 [10] Ko J K, Lamichhane D K, Kim H C, et al. Trends in the prevalences of selected birth defects in Korea (2008-2014). Int J Environ Res Public Health, 2018; 15(5): 923. doi: 10.3390/ijerph15050923 [11] Geneti S A, Dimsu G G, Sori D A, et al. Prevalence and patterns of birth defects among newborns in southwestern Ethiopia: a retrospective study. Pan Afr Med J, 2021; 40: 248. [12] Le M T, Shumate C J, Hoyt A T, et al. The prevalence of birth defects among non-Hispanic Asian/Pacific Islanders and American Indians/Alaska Natives in Texas, 1999-2015. Birth Defects Res, 2019; 111(18): 1380-1388. doi: 10.1002/bdr2.1543 [13] Hao Y, Zhuang D, Jiao X. Seasonal variation of nonsyndromic orofacial clefts in northern Chinese population. J Craniofac Surg, 2022; 33(2): 642-644. doi: 10.1097/SCS.0000000000008185 [14] Liu Q G, Sun J, Xiao X W, et al. Birth defects data from surveillance hospitals in Dalian city, China, 2006-2010. J Matern Fetal Neonatal Med, 2016; 29(22): 3615-3621. doi: 10.3109/14767058.2016.1140136 [15] Wang X, Yue H, Li S, et al. The effects of inositol metabolism in pregnant women on offspring in the north and south of China. Med Sci Monit, 2020; 26: e921088. [16] Baldacci S, Gorini F, Santoro M, et al. Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Esposizione ambientale e individuale e rischio di anomalie congenite: una rassegna delle evidenze epidemiologiche recenti. Epidemiol Prev, 2018; 42(3-4 Suppl 1): 1-34. [17] Candotto V, Oberti L, Gabrione F, et al. Current concepts on cleft lip and palate etiology. J Biol Regul Homeost Agents, 2019; 33(3 Suppl. 1): 145-151. [18] Chen Z, Li S, Guo L, et al. Prenatal alcohol exposure induced congenital heart diseases: From bench to bedside. Birth Defects Res, 2021; 113(7): 521-534. doi: 10.1002/bdr2.1743 [19] Tran D, Maiorana A, Ayer J, et al. Recommendations for exercise in adolescents and adults with congenital heart disease. Prog Cardiovasc Dis, 2020; 63(3): 350-366. doi: 10.1016/j.pcad.2020.03.002 [20] Jiang R, Zhao Y Q, Wang Y C, et al. Discussion on the model of community managemengt of chronic diseases in cold areas. Frigid Zone Medcine, 2021; 1(1): 17-22. doi: 10.2478/fzm-2021-0004 [21] Kodak T, Bergmann S. Autism spectrum disorder: characteristics, associated behaviors, and early intervention. Pediatr Clin North Am, 2020; 67(3): 525-535. doi: 10.1016/j.pcl.2020.02.007 [22] Solmi M, Song M, Yon D K, et al. Incidence, prevalence, and global burden of autism spectrum disorder from 1990 to 2019 across 204 countries. Mol Psychiatry, 2022; 27(10): 4172-4180. doi: 10.1038/s41380-022-01630-7 [23] Sun X, Allison C, Wei L, et al. Autism prevalence in China is comparable to Western prevalence. Mol Autism, 2019; 10: 7. doi: 10.1186/s13229-018-0246-0 [24] Lee B K, Gross R, Francis R W, et al. Birth seasonality and risk of autism spectrum disorder. Eur J Epidemiol, 2019; 34(8): 785-792. doi: 10.1007/s10654-019-00506-5 [25] Sgritta M, Dooling S W, Buffington S A, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron, 2019; 101(2): 246-259. e6. doi: 10.1016/j.neuron.2018.11.018 [26] Siracusano M, Riccioni A, Abate R, et al. Vitamin D deficiency and autism spectrum disorder. Curr Pharm Des, 2020; 26(21): 2460-2474. doi: 10.2174/1381612826666200415174311 [27] Dutheil F, Comptour A, Morlon R, et al. Autism spectrum disorder and air pollution: A systematic review and meta-analysis. Environ Pollut, 2021; 278: 116856. doi: 10.1016/j.envpol.2021.116856 [28] Becerra-Culqui T A, Getahun D, Chiu V, et al. Prenatal influenza vaccination or influenza infection and autism spectrum disorder in offspring. Clin Infect Dis, 2022; 75(7): 1140-1148. doi: 10.1093/cid/ciac101 [29] Dutheil F, Comptour A, Morlon R, et al. Autism spectrum disorder and air pollution: A systematic review and meta-analysis. Environ Pollut, 2021; 278: 116856. doi: 10.1016/j.envpol.2021.116856 [30] Beghi E. The epidemiology of epilepsy. Neuroepidemiology, 2020; 54(2): 185-191. doi: 10.1159/000503831 [31] MacEachern S J, Santoro J D, Hahn K J, et al. Children with epilepsy demonstrate macro- and microstructural changes in the thalamus, putamen, and amygdala. Neuroradiology, 2020; 62(3): 389-397. doi: 10.1007/s00234-019-02332-8 [32] Singh G, Sander J W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav, 2020; 105: 106949. doi: 10.1016/j.yebeh.2020.106949 [33] GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2019; 18(4): 357-375. doi: 10.1016/S1474-4422(18)30454-X [34] Zheng G, Li F, Chen Y, et al. An epidemiological survey of epilepsy in tropical rural areas of China. Epilepsia Open, 2021; 6(2): 323-330. doi: 10.1002/epi4.12476 [35] Syvertsen M, Koht J, Nakken K O. Prevalence and incidence of epilepsy in the Nordic countries. Tidsskr Nor Laegeforen, 2015; 135(18): 1641-1645. doi: 10.4045/tidsskr.15.0454 [36] Chang K C, Wu T H, Fann J C, et al. Low ambient temperature as the only meteorological risk factor of seizure occurrence: A multivariate study. Epilepsy Behav, 2019; 100(Pt A): 106283. [37] Alasfar R H, Isaifan R J. Aluminum environmental pollution: the silent killer. Environ Sci Pollut Res Int, 2021; 28(33): 44587-44597. doi: 10.1007/s11356-021-14700-0 [38] Gunata M, Parlakpinar H, Acet H A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev Neurol (Paris), 2020; 176(3): 148-165. doi: 10.1016/j.neurol.2019.07.025 [39] Guo Y, Du P, Guo L, et al. Alcohol use among patients with epilepsy in western China. A hospital-based study. Epilepsy Behav, 2021; 124: 108302. doi: 10.1016/j.yebeh.2021.108302 [40] Dolbec K, Mick N W. Congenital heart disease. Emerg Med Clin North Am, 2011; 29(4): 811-827, vii. doi: 10.1016/j.emc.2011.08.005 [41] Su Z, Zou Z, Hay S I, et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990-2019: An age-period-cohort analysis for the Global Burden of Disease 2019 study. E Clinical Medicine, 2022; 43: 101249. [42] GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health, 2020; 4(3): 185-200. doi: 10.1016/S2352-4642(19)30402-X [43] Liu F, Yang Y N, Xie X, et al. Prevalence of congenital heart disease in Xinjiang multi-ethnic region of China. PLoS One, 2015; 10(8): e0133961. doi: 10.1371/journal.pone.0133961 [44] Han S, Wei C Y, Hou Z L, et al. Prevalence of congenital heart disease amongst schoolchildren in southwest China. Indian Pediatr, 2020; 57(2): 138-141. doi: 10.1007/s13312-020-1731-z [45] Xia Y Q, Zhao K N, Zhao A D, et al. Associations of maternal upper respiratory tract infection/influenza during early pregnancy with congenital heart disease in offspring: evidence from a case-control study and meta-analysis. BMC Cardiovasc Disord, 2019; 19(1): 277. doi: 10.1186/s12872-019-1206-0 [46] Mills J L, Troendle J, Conley M R, et al. Maternal obesity and congenital heart defects: a population-based study. Am J Clin Nutr, 2010; 91(6): 1543-1549. doi: 10.3945/ajcn.2009.28865 [47] Yang B Y, Qu Y, Guo Y, et al. Maternal exposure to ambient air pollution and congenital heart defects in China. Environ Int, 2021; 153: 106548. doi: 10.1016/j.envint.2021.106548 [48] Soheilirad Z. Folic acid intake in prevention of congenital heart defects: A mini evidence review. Clin Nutr ESPEN, 2020; 38: 277-279. doi: 10.1016/j.clnesp.2020.05.021 [49] Salari N, Darvishi N, Heydari M, et al. Global prevalence of cleft palate, cleft lip and cleft palate and lip: A comprehensive systematic review and meta-analysis. J Stomatol Oral Maxillofac Surg, 2022; 123(2): 110-120. doi: 10.1016/j.jormas.2021.05.008 [50] Zhu Y, Miao H, Zeng Q, et al. Prevalence of cleft lip and/or cleft palate in Guangdong province, China, 2015-2018: a spatio-temporal descriptive analysis. BMJ Open, 2021; 11(8): e046430. doi: 10.1136/bmjopen-2020-046430 [51] Alonso R R H, Brigetty G P S. Analysis of the prevalence and incidence of cleft lip and palate in Colombia. Cleft Palate Craniofac J, 2020; 57(5): 552-559. doi: 10.1177/1055665619886455 [52] Sofianos C, Christofides E A, Phiri S E. Seasonal variation of orofacial clefts. J Craniofac Surg, 2018; 29(2): 368-371. doi: 10.1097/SCS.0000000000004226 [53] Meng Q, Luo J, Li L, et al. Rubella seroprevalence among pregnant women in Beijing, China. BMC Infect Dis, 2018; 18(1): 130. doi: 10.1186/s12879-018-3032-x [54] Auslander A, McKean-Cowdin R, Brindopke F, et al. The role of smoke from cooking indoors over an open flame and parental smoking on the risk of cleft lip and palate: A case-control study in 7 low-resource countries. J Glob Health, 2020; 10(2): 020410. doi: 10.7189/jogh.10.020410 [55] Davies K J M, Richmond S, Medeiros-Mirra R J, et al. The effect of maternal smoking and alcohol consumption on lip morphology. J Orthod, 2022; 49(4): 403-411. doi: 10.1177/14653125221094337 [56] Rezaallah B, Lewis D J, Zeilhofer H F, et al. Risk of cleft lip and/or palate associated with antiepileptic drugs: Postmarketing safety signal detection and evaluation of information presented to prescribers and patients. Ther Innov Regul Sci, 2019; 53(1): 110-119. doi: 10.1177/2168479018761638 [57] Girguis M S, Strickland M J, Hu X, et al. Maternal exposure to traffic-related air pollution and birth defects in Massachusetts. Environ Res, 2016; 146: 1-9. doi: 10.1016/j.envres.2015.12.010 [58] Gmeiner M, Wagner H, Zacherl C, et al. Long-term mortality rates in pediatric hydrocephalus-a retrospective single-center study. Childs Nerv Syst, 2017; 33(1): 101-109. doi: 10.1007/s00381-016-3268-y [59] Tamber M S. Insights into the epidemiology of infant hydrocephalus. Childs Nerv Syst, 2021; 37(11): 3305-3311. doi: 10.1007/s00381-021-05157-0 [60] Liu J, Jin L, Li Z, et al. Prevalence and trend of isolated and complicated congenital hydrocephalus and preventive effect of folic acid in northern China, 2005-2015. Metab Brain Dis, 2018; 33(3): 837-842. doi: 10.1007/s11011-017-0172-4 [61] Abebe M S, Seyoum G, Emamu B, et al. Congenital hydrocephalus and associated risk factors: An institution-based case-control study, Dessie Town, North East Ethiopia. Pediatric Health Med Ther, 2022; 13: 175-182. doi: 10.2147/PHMT.S364447 [62] Liu J, Li Z, Ye R, et al. Folic acid supplementation and risk for congenital hydrocephalus in China. Public Health Nutr, 2021; 24(13): 4238-4244. doi: 10.1017/S136898002100029X [63] Costa L G, Cole T B, Dao K, et al. Developmental impact of air pollution on brain function. Neurochem Int, 2019; 131: 104580. doi: 10.1016/j.neuint.2019.104580 [64] Li T, Fang J, Tang S, et al. PM2.5 exposure associated with microbiota gut-brain axis: Multi-omics mechanistic implications from the BAPE study. Innovation (Camb), 2022; 3(2): 100213. [65] Percy Z, DeFranco E, Xu F, et al. Trimester specific PM2.5 exposure and fetal growth in Ohio, 2007-2010. Environ Res, 2019; 171: 111-118. doi: 10.1016/j.envres.2019.01.031 [66] Wang L, Xiang X, Mi B, et al. Association between early prenatal exposure to ambient air pollution and birth defects: evidence from newborns in Xi'an, China. J Public Health (Oxf), 2019; 41(3): 494-501. doi: 10.1093/pubmed/fdy137 [67] Zhang Q, Sun S, Sui X, et al. Associations between weekly air pollution exposure and congenital heart disease. Sci Total Environ, 2021; 757: 143821. doi: 10.1016/j.scitotenv.2020.143821 [68] Huang C C, Chen B Y, Pan S C, et al. Prenatal exposure to PM2.5 and Congenital Heart Diseases in Taiwan. Sci Total Environ, 2019; 655: 880-886. doi: 10.1016/j.scitotenv.2018.11.284 [69] Li B, Xia M, Zorec R, et al. Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res, 2021; 1752: 147234. doi: 10.1016/j.brainres.2020.147234 [70] Gasmi A, Noor S, Piscopo S, et al. Toxic metal -mediated neurodegradation: A focus on glutathione and GST gene variants. Arch Razi Inst, 2022; 77(2): 525-536. [71] Ramakrishnan R, Stuart A L, Salemi J L, et al. Maternal exposure to ambient cadmium levels, maternal smoking during pregnancy, and congenital diaphragmatic hernia. Birth Defects Res, 2019; 111(18): 1399-1407. doi: 10.1002/bdr2.1555 [72] Wang S Y, Cheng Y Y, Guo H R, et al. Air pollution during pregnancy and childhood autism spectrum disorder in Taiwan. Int J Environ Res Public Health, 2021; 18(18): 9784. doi: 10.3390/ijerph18189784 [73] Huang X, Chen J, Zeng D, et al. The association between ambient air pollution and birth defects in five major ethnic groups in Liuzhou, China. BMC Pediatr, 2021; 21(1): 232. doi: 10.1186/s12887-021-02687-z [74] Roberts D J, Post M D. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol, 2008; 61(12): 1254-1260. doi: 10.1136/jcp.2008.055236 [75] Bedell S, Hutson J, de Vrijer B, et al. effects of maternal obesity and gestational diabetes mellitus on the placenta: Current knowledge and targets for therapeutic interventions. Curr Vasc Pharmacol, 2021; 19(2): 176-192. doi: 10.2174/18756212MTA3qNDApy [76] Huerta-Cervantes M, Peña-Montes D J, López-Vázquez M Á, et al. Effects of gestational diabetes in cognitive behavior, oxidative stress and metabolism on the second-generation off-spring of rats. Nutrients, 2021; 13(5): 1575. doi: 10.3390/nu13051575 [77] Tinker S C, Gilboa S M, Moore C A, et al. Specific birth defects in pregnancies of women with diabetes: National birth defects prevention study, 1997-2011. Am J Obstet Gynecol, 2020; 222(2): 176. e1-176. e11. doi: 10.1016/j.ajog.2019.08.028 [78] Auger N, Fraser W D, Healy-Profitós J, et al. Association between preeclampsia and congenital heart defects. JAMA, 2015; 314(15): 1588-1598. doi: 10.1001/jama.2015.12505 [79] Lin Y W, Lin M H, Pai L W, et al. Population-based study on birth outcomes among women with hypertensive disorders of pregnancy and gestational diabetes mellitus. Sci Rep, 2021; 11(1): 17391. doi: 10.1038/s41598-021-96345-0 [80] Saiyin T, Engineer A, Greco E R, et al. Maternal voluntary exercise mitigates oxidative stress and incidence of congenital heart defects in pre-gestational diabetes. J Cell Mol Med, 2019; 23(8): 5553-5565. doi: 10.1111/jcmm.14439 [81] Samson K L I, Loh S P, Lee S S, et al. Weekly iron-folic acid supplements containing 2.8 mg folic acid are associated with a lower risk of neural tube defects than the current practice of 0.4 mg: a randomised controlled trial in Malaysia. BMJ Glob Health, 2020; 5(12): e003897. doi: 10.1136/bmjgh-2020-003897 [82] Munger R G, Kuppuswamy R, Murthy J, et al. Maternal Vitamin B12 status and risk of cleft lip and cleft palate birth defects in Tamil Nadu State, India. Cleft Palate Craniofac J, 2021; 58(5): 567-576. doi: 10.1177/1055665621998394 [83] Yang J, Kang Y, Chang Q, et al. Maternal Zinc, Copper, and Selenium intakes during pregnancy and congenital heart defects. Nutrients, 2022; 14(5): 1055. doi: 10.3390/nu14051055 [84] Ajmone-Cat M A, De Simone R, Tartaglione A M, et al. Critical role of maternal selenium nutrition in neurodevelopment: Effects on offspring behavior and neuroinflammatory profile. Nutrients, 2022; 14(9): 1850. doi: 10.3390/nu14091850 [85] Perry M F, Mulcahy H, DeFranco E A. Influence of periconception smoking behavior on birth defect risk. Am J Obstet Gynecol, 2019; 220(6): 588. e1-588. e7. doi: 10.1016/j.ajog.2019.02.029 [86] Li J, Du Y J, Wang H L, et al. Association between maternal passive smoking during perinatal period and congenital heart disease in their offspring-based on a case-control study. Zhonghua Liu Xing Bing Xue Za Zhi, 2020; 41(6): 884-889. [87] Meng X, Sun Y, Duan W, et al. Meta-analysis of the association of maternal smoking and passive smoking during pregnancy with neural tube defects. Int J Gynaecol Obstet, 2018; 140(1): 18-25. doi: 10.1002/ijgo.12334 [88] Shi J, Tian Y, Lei Y, et al. Active and passive maternal smoking during pregnancy and risk of having a child with polydactyly: a case-control study. Zhonghua Liu Xing Bing Xue Za Zhi, 2018; 39(11): 1482-1485. [89] Yin C, Cai H, Yang D, et al. Cigarette smoke induced neural tube defects by down-regulating noggin expression. Birth Defects Res, 2021; 113(1): 5-13. doi: 10.1002/bdr2.1804 [90] Luderer M, Ramos Quiroga J A, Faraone S V, et al. Alcohol use disorders and ADHD. Neurosci Biobehav Rev, 2021; 128: 648-660. doi: 10.1016/j.neubiorev.2021.07.010 [91] Strandberg-Larsen K, Skov-Ettrup L S, Grønbaek M, et al. Maternal alcohol drinking pattern during pregnancy and the risk for an offspring with an isolated congenital heart defect and in particular a ventricular septal defect or an atrial septal defect. Birth Defects Res A Clin Mol Teratol, 2011; 91(7): 616-622. doi: 10.1002/bdra.20818 [92] Barinaga M. Neurobiology. A new clue to how alcohol damages brains. Science, 2000; 287(5455): 947-948. doi: 10.1126/science.287.5455.947 [93] Chen Z, Li S, Guo L, et al. Prenatal alcohol exposure induced congenital heart diseases: From bench to bedside. Birth Defects Res, 2021; 113(7): 521-534. doi: 10.1002/bdr2.1743 [94] Messinger C J, Lipsitch M, Bateman B T, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr, 2020; 174(12): 1159-1167. doi: 10.1001/jamapediatrics.2020.3009 [95] Zhang L, Wang X, Liu M, et al. The epidemiology and disease burden of congenital TORCH infections among hospitalized children in China: A national cross-sectional study. PLoS Negl Trop Dis, 2022; 16(10): e0010861. doi: 10.1371/journal.pntd.0010861 [96] Bookstaver P B, Bland C M, Griffin B, et al. A review of antibiotic use in pregnancy. Pharmacotherapy, 2015; 35(11): 1052-1062. doi: 10.1002/phar.1649 [97] Hamilton S T, Scott G, Naing Z, et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One, 2012; 7(12): e52899. doi: 10.1371/journal.pone.0052899 [98] Zhu P, Zhang Z H, Huang X F, et al. Cold exposure promotes obesity and impairs glucose homeostasis in mice subjected to a highfat diet. Mol Med Rep, 2018; 18(4): 3923-3931. -


 投稿系统
投稿系统


 下载:
下载: