Protective mechanism of Ramulus Mori (Sangzhi) Alkaloids on T2DM combined with MASLD by hepatic lipid metabolism and gut microbiota analyses
doi: 10.1515/fzm-2025-0026
-
Abstract:
Background and objective Both type 2 diabetes mellitus (T2DM) and metabolic dysfunction-associated steatotic liver disease (MASLD) are known to be influenced by environmental and lifestyle factors. Ramulus Mori (Sangzhi) alkaloids (SZ-A) are effective hypoglycemic agents. Recent studies suggest that SZ-A may improve T2DM, MASLD, and metabolic syndrome, but the underlying mechanisms remain unclear. This study aimed to investigate whether SZ-A can modulate hepatic lipid metabolism and gut microbiota in a mouse model of T2DM combined with MASLD. Methods A combined T2DM-MASLD mouse model was established using a high-fat diet and streptozotocin injection. Liver morphology and histology were assessed using a portable small-animal ultrasound imaging system, hematoxylin and eosin (H&E) staining, and Oil Red O staining. Serum levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured using standard assay kits. Gut microbiota composition was analyzed by 16S rRNA sequencing, and hepatic lipid metabolites were profiled using liquid chromatographymass spectrometry (LC-MS)MS. Results SZ-A improved liver function by ameliorating morphological and structural abnormalities, reducing lipid droplet accumulation, and lowering serum levels of TG, TC, LDL, ALT, and AST. It also led to decreased hepatic ultrasound echo intensity compared to the kidney. Additionally, SZ-A helped restore gut microbiota balance, including a partial reversal of the Firmicutes/Bacteroidetes ratio. Lipidomic analysis revealed that SZ-A downregulated most TG and diglycerides (DG), while upregulating phosphatidylcholine (PC) and phosphatidylethanolamine (PE) in the model group. Conclusions SZ-A partially alleviates liver injury in T2DM-MASLD mice by modulating hepatic lipid metabolism and gut microbiota composition. -
Key words:
- Ramulus Mori (Sangzhi) Alkaloids /
- gut microbiota /
- lipidomics analysis
-
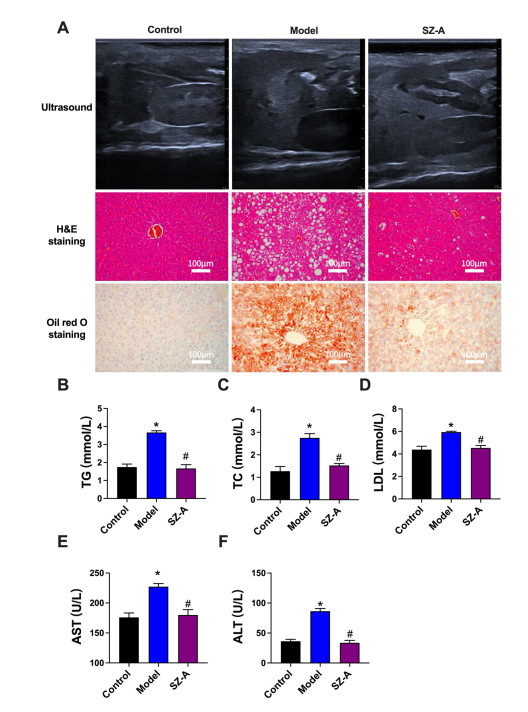
Figure 1. Effect of SZ-A on liver function in type 2 diabetes mellitus (T2DM) combined with metabolic dysfunction-associated steatotic liver disease (MASLD) mice
The function of liver was detected by Ultrasound, H&E staining, and Oil red O staining assays. (B-D) The changes of TG (B), TC (C) and LDL (D) in serum among different groups. (E and F) Effect of SZ-A on alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in different groups. vs. Control, *P < 0.05. vs. Model #P < 0.05. T2DM, type 2 diabetes mellitus; MASLD, metabolic dysfunction-associated steatotic liver disease; TG, triglycerides; TC, total cholesterol; LDL, low-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Figure 2. Effect of SZ-A on gut microbiota by alpha diversity analysis in type 2 diabetes mellitus (T2DM) combined with metabolic dysfunction-associated steatotic liver disease (MASLD) mice
(A and B) Top 10 species at the phylum level (A) and at the genus level (B). (C-F) Alpha diversity indices were analyzed, including Chao1 (C), Dominance (D), pielou_e (E) and Shannon (F) in different groups. N = 5 in each group. vs. Control, *P < 0.05. vs. Model #P < 0.05. **P < 0.01; ***P < 0.001.
Figure 3. Effect of SZ-A on gut microbiota by beta diversity analysis in type 2 diabetes mellitus (T2DM) combined with metabolic dysfunction-associated steatotic liver disease (MASLD) mice
(A) Principal co-ordinates analysis (PCoA) analysis. (B) Unweighted pair-group method with arithmetic means (UPGMA) cluster tress analysis. (C and D) Linear discriminant analysis (LDA) Effect Size (C) and branching diagram (D). (E) Venn diagram showed the overlap or unique microflora among different groups. (F-H) Effect of SZ-A on Firmicutes (F), Bacteroidetes (G) and ratio of Firmicutes to Bacteroidetes (H). N = 5 in each group. vs. Control, *P < 0.05. vs. Model #P < 0.05.
Figure 4. Effect of SZ-A on hepatic lipid metabolites by multivariate statistical analysis in type 2 diabetes mellitus (T2DM) combined with metabolic dysfunction-associated steatotic liver disease (MASLD) mice
(A) Principal co-ordinates analysis (PCoA) analysis between control and model group. (B) PCA analysis between model and SZ-A group. (C) Partial least squares discrimination analysis (PLSDA) analysis between control and model group. (D) PLSDA analysis between model and SZ-A group. (E) Volcanic map between control and model group. (F) Volcanic map between model and SZ-A group. (G) Venn diagram among different groups.
Figure 5. Effect of SZ-A on hepatic lipid metabolism pathway in T2DM combined with MASLD mice
(A) Heap maps among different groups. (B) Top changed metabolites between control and model group. (C) Top changed metabolites between model and SZ-A group. (D) The changes of TG, DG, PC and PE among different groups. (E) KEGG analysis between control and model group. (F) KEGG analysis between model and SZ-A group. T2DM, type 2 diabetes mellitus; MASLD, metabolic dysfunction-associated steatotic liver disease; TG, triglycerides; DG, diacylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; KEGG, Kyoto encyclopedia of genes and genomes.
Figure 6. Effect of SZ-A on the correlation of hepatic lipid metabolites and gut microbiota
(A) The correlation of hepatic lipid metabolites and gut microbiota between control and model group. (B) The correlation of hepatic lipid metabolites and gut microbiota between model and SZ-A group. (C) The correlation of gut microbiota serum and biochemistry analysis between control and model group. (D) The correlation of gut microbiota serum and biochemistry analysis between model and SZ-A group. (E) The correlation of hepatic lipid metabolites and serum biochemistry analysis between control and model group. (F) The correlation of hepatic lipid metabolites and serum biochemistry analysis between model and SZ-A group.
-
[1] Ferguson D, Finck B N. Emerging therapeutic approaches for the treatment of MASLD and type 2 diabetes mellitus. Nat Rev Endocrinol, 2021; 17(8): 484-495. doi: 10.1038/s41574-021-00507-z [2] Targher G, Corey K E, Byrne C D, et al. The complex link between MASLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol, 2021; 18(9): 599-612. doi: 10.1038/s41575-021-00448-y [3] Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol, 2022; 10(4): 284-296. doi: 10.1016/S2213-8587(22)00003-1 [4] En Li Cho E, Ang C Z, et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Gut, 2023; 72(11): 2138-2148. doi: 10.1136/gutjnl-2023-330110 [5] Tanase D M, Gosav E M, Costea C F, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (MASLD). J Diabetes Res, 2020; 2020: 3920196. doi: 10.1155/2020/3920196 [6] Poustchi H, Alaei-Shahmiri F, Aghili R, et al. Hepatic steatosis and fibrosis in type 2 diabetes: A risk-based approach to targeted screening. Arch Iran Med, 2021; 24(3): 177-186. doi: 10.34172/aim.2021.28 [7] Hong S H, Choi K M. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci, 2020; 21(2): 494. doi: 10.3390/ijms21020494 [8] Zhou M, Liu X, Wu Y, et al. Liver lipidomics analysis revealed the protective mechanism of Zuogui Jiangtang Qinggan Formula in type 2 diabetes mellitus with non-alcoholic fatty liver disease. J Ethnopharmacology, 2024; 329: 118160. doi: 10.1016/j.jep.2024.118160 [9] Alfadda A A, Almaghamsi A M, Sherbeeni S M, et al. Alterations in circulating lipidomic profile in patients with type 2 diabetes with or without non-alcoholic fatty liver disease. Front Mol Biosci, 2023; 10: 1030661. doi: 10.3389/fmolb.2023.1030661 [10] Schoeler M, Ellero-Simatos S, Birkner T, et al. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat Commun, 2023; 14(1): 5329. doi: 10.1038/s41467-023-41074-3 [11] An X, Yang X, Ding X, et al. Ramulus Mori (Sangzhi) alkaloids tablets for diabetes mellitus: A regulatory perspective. Fitoterapia, 2023; 166: 105444. doi: 10.1016/j.fitote.2023.105444 [12] Sun Q W, Lian C F, Chen Y M, et al. Ramulus Mori (Sangzhi) alkaloids ameliorate obesity-linked adipose tissue metabolism and inflammation in mice. Nutrients, 2022; 14(23): 5050. doi: 10.3390/nu14235050 [13] Yang Y, Wu L, Lv Y, et al. LC-MS/MS based untargeted lipidomics uncovers lipid signatures of late-onset preeclampsia. Biochimie, 2023; 208: 46-55. doi: 10.1016/j.biochi.2022.12.002 [14] Bailey L S, Huang F, Gao T, et al. Characterization of glycosphingolipids and their diverse lipid forms through two-stage matching of LC-MS/MS Spectra. Anal Chem, 2021; 93(6): 3154-3162. doi: 10.1021/acs.analchem.0c04542 [15] Liu D, Ye J, Yan Y, et al. Ramulus Mori (Sangzhi) alkaloids regulates gut microbiota disorder and its metabolism profiles in obese mice induced by a high-fat diet. Front Pharmacol, 2023; 14: 1166635. doi: 10.3389/fphar.2023.1166635 [16] Liu W, Xu S, Zhang B, et al. Ramulus Mori (Sangzhi) alkaloids alleviate diabetic nephropathy through improving gut microbiota disorder. Nutrients, 2024; 16(14): 2346. doi: 10.3390/nu16142346 [17] Wang F, Xu S J, Ye F, et al. Integration of transcriptomics and lipidomics profiling to reveal the therapeutic mechanism underlying Ramulus Mori (Sangzhi) Alkaloids for the treatment of liver lipid metabolic disturbance in high-fat-diet/streptozotocin-induced Diabetic Mice. Nutrients, 2023; 15(18): 3914. doi: 10.3390/nu15183914 -


 投稿系统
投稿系统


 下载:
下载: