Circular RNA signatures in vestibular migraine and migraine from cold regions: Preliminary mechanistic insights
doi: 10.1515/fzm-2025-0022
-
Abstract:
Background Vestibular migraine (VM) is a common disorder characterized by recurrent dizziness or vertigo, often aggravated by cold exposure. This study aimed to identify differentially expressed circular RNAs (circRNAs) in cold-region VM and explore the underlying molecular mechanisms. Methods Peripheral blood samples from long-term residents of Heilongjiang Province profiled by circRNA microarray, and differentially expressed circRNAs were validated by quantitative reverse transcription polymerase chain reaction (qRT-PCR). A competing endogenous RNA (ceRNA) network and enriched pathways were inferred by bioinformatics. A VM-like mouse model was established using nitroglycerin (NTG) and kainic acid (KA) and confirmed by behavioral testing and western blot. The hsa_circ_0003201/miR-31-5p/triggering receptor expressed on myeloid cells 2 (TREM2) axis and related pathways were examined in clinical samples and in the trigeminal nucleus caudalis (TNC) and vestibular nuclei (VN) of mice using qRT-PCR, enzyme-linked immunosorbent assay (ELISA), and western blot. CircRNA microarray profiling also compared expression patterns between VM and migraine patients. Results Hsa_circ_0003201 was significantly upregulated in cold-region VM patients. Bioinformatic analyses revealed that hsa_circ_0003201 may regulate the miR-31-5p/TREM2 axis and be associated with phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling, pyruvate metabolism, and transient receptor potential (TRP) pathways. Clinical validation confirmed increased hsa_circ_0003201 and TREM2 and decreased miR-31-5p. VM-like mice exhibited central sensitization and vestibular dysfunction, with increased TREM2, decreased miR-31-5p, and PI3K/AKT activation in the TNC and VN. Comparative circRNA analysis between VM and migraine patients indicated distinct expression patterns. Conclusion Hsa_circ_0003201 shows potential as a diagnostic biomarker for cold-region VM, and the hsa_circ_0003201/miR-31-5p/TREM2 axis may contribute to pathogenesis through PI3K/AKT signaling, pyruvate metabolism, and TRP-related pathways. -
Key words:
- circRNA /
- vestibular /
- migraine /
- cold regions
-
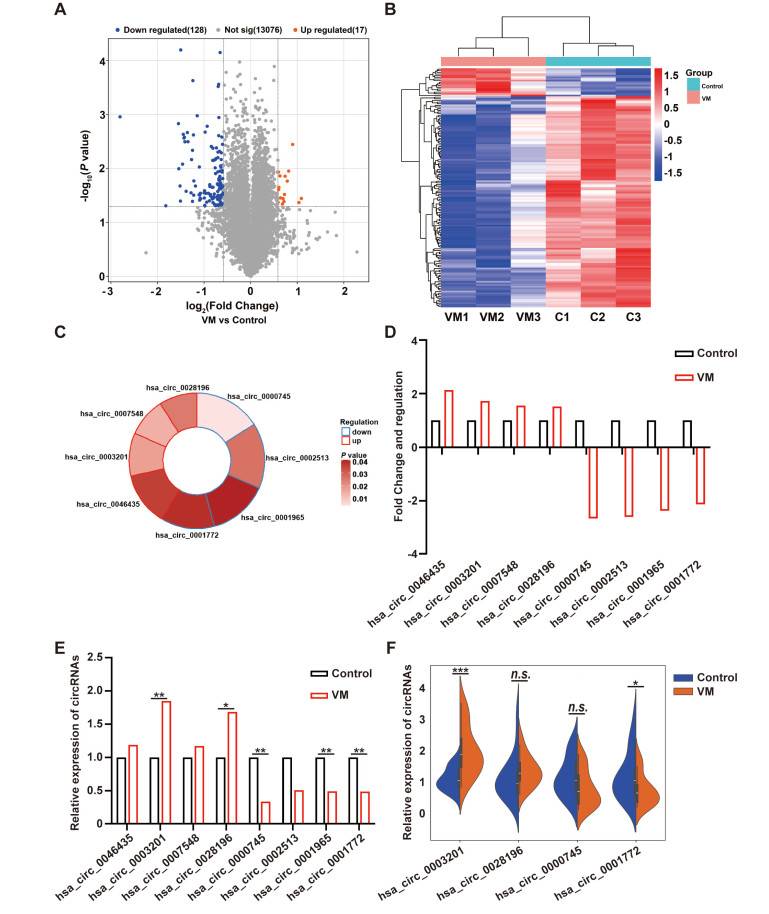
Figure 1. Screening and validation of differentially expressed circRNAs in vestibular migraine (VM) patients
(A-B) Volcano plot and hierarchical clustering of differentially expressed circRNAs between VM patients and healthy controls. (C) Top 4 upregulated and top 4 downregulated circRNAs. (D) Microarray expression patterns of the 8 candidate circRNAs (N = 3/group). (E) Validation of the 8 candidate circRNAs by quantitative reverse transcription polymerase chain reaction (qRT-PCR) (N = 3/group). (F) Relative expression of the 4 selected circRNAs in VM patients measured by qRT-PCR (N = 20/group). The data are presented as mean ± SD. Student's t-test (E and F). *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significant.
Figure 2. Construction of the vestibular migraine (VM)-associated hsa_circ_0003201/miR-31-5p/triggering receptor expressed on myeloid cells 2 (TREM2) axis
(A) Gene ontology (GO) functional enrichment analysis of hsa_circ_0003201-related genes. (B) hsa_circ_0003201-based ceRNA network related to VM, with the key hsa_circ_0003201/miR-31-5p/TREM2 axis identified in this study highlighted in red. (C) Top 20 enriched pathways from Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis, with the pathways relevant to this study highlighted in red. (D) The transient receptor potential (TRP) channel pathways (hsa04270, hsa04750). (E-F) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) detection of hsa_circ_0003201 and hsa-miR-31-5p expression levels (N = 30/ group). (G) Enzyme-linked immunosorbent assay (ELISA) analysis of TREM2 expression levels (N = 30/group). The data are presented as mean ± SD. Student's t-test (E-G). *P < 0.05, ***P < 0.001.
Figure 3. Establishment of vestibular migraine (VM)-like mouse model
(A) Flowchart of mouse model induction and behavioral assessments. (B) The periorbital mechanical thresholds were assessed using von Frey filaments (N = 8/group). (C-D) Balance beam walking time and vestibular dysfunction tests of mice (N = 8/group). (E) Western blot analysis of calcitonin gene related peptide (CGRP) and c-Fos protein expression in the trigeminal nucleus caudalis (TNC) (N = 6/group). (F) CGRP and c-Fos protein levels in the vestibular nuclei (VN) analyzed by western blot (N = 6/group). The data are shown as mean ± SD. The P values were calculated using two-way analysis of variance (ANOVA) followed by Tukey's post hoc tests (B and C) and one-way ANOVA (D-F). *P < 0.05, **P < 0.01, ***P < 0.001. M, Migraine.
Figure 4. Altered expression of triggering receptor expressed on myeloid cells 2 (TREM2) and miR-31-5p with activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway in the trigeminal nucleus caudalis (TNC) and vestibular nuclei (VN) of vestibular migraine (VM)-like mice
(A) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of mmu-miR-31-5p expression in the TNC. (B) Western blot analysis of TREM2 in the TNC of each group. (C) qRT-PCR validation of mmu-miR-31-5p in VN. (D) TREM2 protein levels in the VN analyzed by western blot. (E-F) Western blot analysis of PI3K/ AKT pathway proteins in the TNC and VN of mice. N = 6/group. The data are presented as mean ± SD. The P values were calculated using one-way analysis of variance (ANOVA) (A-F). *P < 0.05, **P < 0.01. M, Migraine.
Figure 5. Screening of differentially expressed circRNAs in vestibular migraine (VM) and Migraine (M) patients
(A) Volcano plot of differentially expressed circRNAs between VM and M patients. (B) Top 4 upregulated and top 4 downregulated circRNAs. (C-D) Gene ontology (GO) functional and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis of differentially expressed circRNAs in VM and M patients.
Table 1. Comparison of age and sex among enrolled patients
Control (N = 53) VM (N = 53) P value Age 52.77 ± 11.84 56.25 ± 10.16 0.1116 Sex 0.5260 Male 7 4 Female 46 49 Table 2. Sequences of primers used in quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Name Sequence β-actin F: 5' GTGGCCGAGGACTTTGATTG3' R: 5' CCTGTAACAACGCATCTCATATT3' hsa_circ_0046435 F: 5' TCTAGGTCAACACCAGCTACCA3' R: 5' GCGGCACTTTGATGAAATAAC3' hsa_circ_0003201 F: 5' TCTGAAGTGGAGAACGAAGAAAT 3' R: 5' CTTCATACTGCTGTCTGTGCTTC 3' hsa_circ_0007548 F: 5' CTACTCTCTGCTTCCCTAGACAAC 3' R: 5' TTTGATGTGACGTAAGTTTTTGC 3' hsa_circ_0028196 F: 5' CCCTTGATGCCATTCTAGTGA 3' R: 5' CACAGCATTCCGATATTCCTT 3' hsa_circ_0000745 F: 5' TTACTAAAGGCAAACGGTGAA 3' R: 5' GAGTGGGAGTGTTGGAAGAAG 3' hsa_circ_0002513 F: 5' TCAAAGCTGCCCAATGATCTG 3' R: 5' GGAAAGTGTAGTTGCCCTCCC 3' hsa_circ_0001965 F: 5' ACCAGTTAATAGCACCAGCTGA 3' R: 5' GACGACGAGACAACAGGAATG 3' hsa_circ_0001772 F: 5' GAACCTTTAGATGAATTTACATGAA 3' R: 5' CCGACTGATTCTTTTTGCC 3' hsa-miR-31-5p and mmu-miR-31-5p were designed and synthesized by RiboBio (Guangzhou, China). Table 3. Antibodies for western blot
Name Manufacturer Catalog Number Dilution CGRP Abcam ab283568 1∶1000 c-Fos CST 2250 1∶1000 GAPDH Immunoway YN5585 1∶10000 TREM2 MCE HY-P80920 1∶1000 β-Actin Affinity T0022 1∶10000 PI3K Immunoway YT6156 1∶2000 P-AKT Immunoway YP0006 1∶2000 AKT Immunoway YT0185 1∶2000 Goat Anti Rabbit IgG (H+L) Immunoway RS0002 1∶10000 Goat Anti Mouse ABclonal AS003 1:10000 IgG (H+L) CGRP, Calcitonin gene-related peptide; GADPH, glyceraldehyde-3-phosphate dehydrogenase; TREM2, triggering receptor expressed on myeloid cells 2; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; IgG, immunoglobulin G. Table 4. Top 4 upregulated and top 4 downregulated circRNAs in vestibular migraine (VM) patients
Circbase ID Chrom P value Fold change (abs) Regulation circRNA_type Gene symbol hsa_circ_0046435 chr17 0.035982 2.125836 Up Exonic FOXK2 hsa_circ_0003201 chr4 0.017203 1.724902 Up Exonic TBC1D14 hsa_circ_0007548 chr16 0.013781 1.546976 Up Exonic RFWD3 hsa_circ_0028196 chr12 0.024714 1.514584 Up Exonic ANAPC7 hsa_circ_0000745 chr17 0.003209 2.660764 Down Exonic SPECC1 hsa_circ_0002513 chr11 0.026517 2.612719 Down Exonic PICALM hsa_circ_0001965 chr3 0.040671 2.376915 Down Exonic PHC3 hsa_circ_0001772 chr7 0.038666 2.136985 Down Exonic RBM33 Table 5. Top 4 upregulated and top 4 downregulated circRNAs between vestibular migraine (VM) and Migraine (M) patients
Circbase ID Chrom P value Fold change (abs) Regulation circRNA_type Gene symbol hsa_circ_0057691 Chr2 0.021791 1.976748 Up Exonic SATB2 hsa_circ_0007390 Chr15 0.000234 1.901624 Up Exonic NPTN hsa_circ_0055451 Chr2 0.007965 1.846619 Up Exonic ST3GAL5 hsa_circ_0007418 chr1 0.005496 1.783638 Up Exonic ZBTB40 hsa_circ_0068655 Chr3 0.001609 14.468478 Down Exonic UBXN7 hsa_circ_0000542 Chr14 0.017122 3.332816 Down Exonic ARID4A hsa_circ_0001534 Chr5 0.001058 1.638913 Down Exonic FAM13B hsa_circ_0000829 Chr18 0.013600 1.510387 Down Exonic SPIRE1 -
[1] Dong L, Dong W, Jin Y, et al. The global burden of migraine: A 30-year trend review and future projections by age, sex, country, and region. Pain Ther, 2025; 14(1): 297-315. doi: 10.1007/s40122-024-00690-7 [2] GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2019; 18(5): 459-480. doi: 10.1016/S1474-4422(18)30499-X [3] Formeister E J, Rizk H G, Kohn M A, et al. The epidemiology of vestibular migraine: A population-based survey study. Otol Neurotol, 2018; 39(8): 1037-1044. doi: 10.1097/MAO.0000000000001900 [4] Furman J M, Marcus D A, Balaban C D. Vestibular migraine: Clinical aspects and pathophysiology. Lancet Neurol, 2013; 12(7): 706-715. doi: 10.1016/S1474-4422(13)70107-8 [5] Ye S, Gao Y, Lin Y, et al. Ambient nitrogen dioxide, temperature exposure, and migraine incidence: A large prospective cohort study. Headache, 2025; 65(8): 40859714. doi: 10.1111/head.15037 [6] Li W, Bertisch S M, Mostofsky E, et al. Weather, ambient air pollution, and risk of migraine headache onset among patients with migraine. Environ Int, 2019; 132: 105100. doi: 10.1016/j.envint.2019.105100 [7] Scheidt J, Koppe C, Rill S, et al. Influence of temperature changes on migraine occurrence in Germany. Int J Biometeorol, 2013; 57(4): 649-654. doi: 10.1007/s00484-012-0582-2 [8] Zhang J, Luo Z, Zheng Y, et al. CircRNA as an Achilles heel of cancer: Characterization, biomarker and therapeutic modalities. J Transl Med, 2024; 22(1): 752. doi: 10.1186/s12967-024-05562-4 [9] Misir S, Wu N, Yang B B. Specific expression and functions of circular RNAs. Cell Death Differ, 2022; 29(3): 481-491. doi: 10.1038/s41418-022-00948-7 [10] Yang L, Han B, Zhang Z, et al. Extracellular vesicle-mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation, 2020; 142(6): 556-574. doi: 10.1161/CIRCULATIONAHA.120.045765 [11] García-Domínguez M. Role of ncRNAs in the development of chronic pain. Noncoding RNA, 2025; 11(4): 51. doi: 10.3390/ncrna11040051 [12] Lin J, Shi S, Chen Q, et al. Differential expression and bioinformatic analysis of the circRNA expression in migraine patients. Biomed Res Int, 2020; 2020: 4710780. doi: 10.1155/2020/4710780 [13] Song B, Fu J, Cheng J, et al. Circular RNA circFat3 as a biomarker for construction of postmortem interval Estimation models in mouse brain tissues at multiple temperatures. Sci Rep, 2025; 15(1): 21577. doi: 10.1038/s41598-025-07998-0 [14] Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia, 2013; 33(9): 629-808. doi: 10.1177/0333102413485658 [15] Chen H, Tang X, Li J, et al. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine. J Headache Pain, 2022; 23(1): 1. doi: 10.1186/s10194-021-01374-9 [16] Gaboyard-Niay S, Travo C, Saleur A, et al. Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammalian vestibule: A rat model of vertigo symptoms. Dis Model Mech, 2016; 9(10): 1181-1192. doi: 10.1242/dmm.024521 [17] Chaplan S R, Bach F W, Pogrel J W, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods, 1994; 53(1): 55-63. doi: 10.1016/0165-0270(94)90144-9 [18] Cohen J M, Bigal M E, Newman L C. Migraine and vestibular symptoms-identifying clinical features that predict vestibular migraine. Headache, 2011; 51(9): 1393-1397. doi: 10.1111/j.1526-4610.2011.01934.x [19] Saldaña-Ruíz S, Soler-Martín C, Llorens J. Role of CYP2E1-mediated metabolism in the acute and vestibular toxicities of nineteen nitriles in the mouse. Toxicol Lett, 2012; 208(2): 125-132. doi: 10.1016/j.toxlet.2011.10.016 [20] Li H, Huang Y, Chen Q, et al. Effect of activated autophagy on an animal model of vestibular migraine-like attacks. Exp Neurol, 2025; 392: 115366. doi: 10.1016/j.expneurol.2025.115366 [21] Wen Q, Wang Y, Pan Q, et al. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J Neuroinflammation, 2021; 18(1): 287. doi: 10.1186/s12974-021-02342-5 [22] Ashina M. Migraine. N Engl J Med, 2020; 383(19): 1866-1876. doi: 10.1056/NEJMra1915327 [23] Espinosa-Sanchez J M, Lopez-Escamez J A. New insights into pathophysiology of vestibular migraine. Front Neurol, 2015; 6: 12. doi: 10.3389/fneur.2015.00012 [24] Muhammed T M, Jasim S A, Uthirapathy S, et al. CircRNA-based therapeutics: A new frontier in neuroinflammation treatment. Mol Neurobiol, 2025; Published online: 40745517. [25] Chen J, Xiao S, Cui X, et al. Circ_0049472 downregulation relieves Amyloid-β-induced neuronal injury by modulating PDE4A expression via targeting miR-22-3p in Alzheimer's disease. Metab Brain Dis, 2025; 40(7): 252. doi: 10.1007/s11011-025-01652-4 [26] Liu Q, Chen M, Cai X, et al. Role of circ_0002590 in neuroinflammation via the miR-1184/NLRP1 axis in painful diabetic neuropathy. Exp Clin Endocrinol Diabetes, 2025; 133(8): 415-424. doi: 10.1055/a-2676-1452 [27] Zuo L, Xie J, Liu Y, et al. Down-regulation of circular RNA CDC14A peripherally ameliorates brain injury in acute phase of ischemic stroke. J Neuroinflammation, 2021; 18(1): 283. doi: 10.1186/s12974-021-02333-6 [28] Dziadkowiak E, Baczyńska D, Wieczorek M, et al. MiR-31-5p as a potential circulating biomarker and tracer of clinical improvement for chronic inflammatory demyelinating polyneuropathy. Oxid Med Cell Longev, 2023; 2023: 2305163. doi: 10.1155/2023/2305163 [29] Liu Y, Wang L, Zhou C, et al. MiR-31-5p regulates the neuroinflammatory response via TRAF6 in neuropathic pain. Biol Direct, 2024; 19(1): 10. doi: 10.1186/s13062-023-00434-1 [30] Zhao J, Xu H, Duan Z, et al. MiR-31-5p regulates 14-3-3 ɛ to inhibit prostate cancer 22RV1 cell survival and proliferation via PI3K/AKT/Bcl-2 signaling pathway. Cancer Manag Res, 2020; 12: 6679-6694. doi: 10.2147/CMAR.S247780 [31] Wang W, Tang L, Li Q, et al. Overexpression of miR-31-5p inhibits human chordoma cells proliferation and invasion by targeting the oncogene c-Met through suppression of AKT/PI3K signaling pathway. Int J Clin Exp Pathol, 2017; 10(7): 8000-8009. [32] Wang Y, Cella M, Mallinson K, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell, 2015; 160(6): 1061-1071. doi: 10.1016/j.cell.2015.01.049 [33] Bayraktaroglu I, Ortí-Casañ N, Van Dam D, et al. Systemic inflammation as a central player in the initiation and development of Alzheimer's disease. Immun Ageing, 2025; 22(1): 33. doi: 10.1186/s12979-025-00529-5 [34] Fan Z, Su D, Li Z C, et al. Metformin attenuates central sensitization by regulating neuroinflammation through the TREM2-SYK signaling pathway in a mouse model of chronic migraine. J Neuroinflammation, 2024; 21(1): 318. doi: 10.1186/s12974-024-03313-2 [35] Chen S, Peng J, Sherchan P, et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation, 2020; 17(1): 168. doi: 10.1186/s12974-020-01853-x [36] Han X, Cheng X, Xu J, et al. Activation of TREM2 attenuates neuroinflammation via PI3K/Akt signaling pathway to improve postoperative cognitive dysfunction in mice. Neuropharmacology, 2022; 219: 109231. doi: 10.1016/j.neuropharm.2022.109231 [37] Nassini R, Materazzi S, Benemei S, et al. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol, 2014; 167: 1-43. doi: 10.1007/112_2014_18 [38] Yue L, Xu H. TRP channels in health and disease at a glance. J Cell Sci, 2021; 134(13): jcs258372. doi: 10.1242/jcs.258372 [39] Strassman A, Mason P, Moskowitz M, et al. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res, 1986; 379(2): 242-250. doi: 10.1016/0006-8993(86)90777-8 [40] Weng J, Liu Q, Li C, et al. TRPA1-PI3K/Akt-OPA1-ferroptosis axis in ozone-induced bronchial epithelial cell and lung injury. Sci Total Environ, 2024; 918: 170668. doi: 10.1016/j.scitotenv.2024.170668 [41] Luo Q Q, Wang B, Chen X, et al. Acute stress induces visceral hypersensitivity via glucocorticoid receptor-mediated membrane insertion of TRPM8: Involvement of a non-receptor tyrosine kinase Pyk2. Neurogastroenterol Motil, 2020; 32(10): 1514-1528. doi: 10.1111/nmo.13877 [42] Kelbert J, Tobin J A. The effect of ambient temperature on migraine disease: A scoping review. Brain Behav, 2025; 15(8): e70708. doi: 10.1002/brb3.70708 [43] Zhai Q, Chen Q, Zhang N, et al. Exploring vestibulocerebellum-vestibular nuclei-spinal trigeminal nucleus causals communication and [44] TRPV2 ion channel in a mouse model of vestibular migraine. J Headache Pain, 2025; 26(1): 47. [45] Hanan M, Soreq H, Kadener S. CircRNAs in the brain. RNA Biol, 2017; 14(8): 1028-1034. doi: 10.1080/15476286.2016.1255398 -


 投稿系统
投稿系统


 下载:
下载: