Rewarming strategies for cryopreservation: Technological challenges and opportunities in energy conversion
doi: 10.1515/fzm-2025-0010
-
Abstract: Cryopreservation of living cells and tissues plays a vital role in biomedical research, clinical applications, biotechnology innovation, the development of new vaccines and drugs, and the conservation of endangered species. While significant technological breakthroughs have been achieved in cooling methods—particularly through vitrification for large tissue and organs—the lack of optimal rewarming technology remains a key obstacle to successful cryopreservation, especially for larger samples such as tissues and organs. The primary challenges during the warming process include non-uniformity heating and insufficient rewarming rates, which can lead to thermal stress-induced structural damage and lethal ice recrystallization, ultimately compromising the integrity and functionality of biological materials. In recent years, various advanced warming techniques have emerged, employing different energy conversion approaches to achieve volumetric heating while minimizing the risk of overheating. These techniques involve thermal, mechanical-thermal, and electromagnetic-thermal energy conversions. However, each method presents its own limitation. This review aims to summarize recent advancements in rewarming technologies for cryopreservation, with a focus on their mechanisms, applications, and the key challenges that must be addressed to enable broader adoption in medical and commercial contexts.
-
Key words:
- cryopreservation /
- rewarming technology /
- energy conservation /
- electromagnetic
-
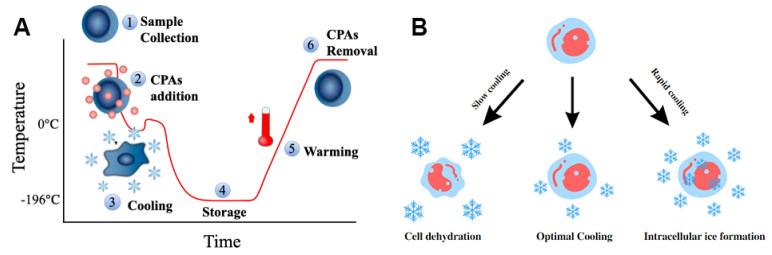
Figure 1. Cryopreservation standard procedures and Peter Mazur's "two-factor" hypothesis
(A) Major steps of cryopreservation. The sample temperature is initially lowered to 0 ℃ to allow for the addition of cryoprotective agents (CPAs), followed by further cooling to reach the target cryogenic temperature. Storage requires proper moisture control and maintenance of a stable low temperature. Rewarming then returns the sample from ultra-low temperatures to 0 ℃, after which CPAs are removed. Finally, sample functionality and metabolic activity are assessed before future application. (B) Peter Mazur's "Two-factor" Hypothesis. During slow cooling, cells undergo dehydration due to the solution effect. Conversely, rapid cooling can lead to intracellular ice formation, which is lethal to cells. Therefore, an optimal, cell-type-specific cooling rate is required to avoid both intracellular ice formation and excessive dehydration.
Figure 2. Energy conversion sources and methods used in cryopreservation rewarming technologies
Three primary categories of energy sources-thermal, mechanical, and electromagnetic-are employed to achieve rapid and uniform heating. Each category involves distinct heating mechanisms and has specific applications in cryopreservation rewarming.
Figure 3. Illustration of the conventional heating method in cryopreservation
(A) Dry thawing utilizing a conduction heat transfer mechanism, where heated plates warm the sample via direct contact. (B) Water bath rewarming, where the sample is immersed in warm water to enable heat transfer through conduction and convection. (C) Hot moving fluid rewarming (typically heated air), demonstrating a convection heat transfer mechanism from left to right.
Figure 4. Heating mechanism of laser rewarming and its setup
(A) The mechanism of laser rewarming with nanorods or nanoparticles infused into the cryopreserved sample. The laser is directed onto the cryopreserved sample, such as cells, and dispersed by the nanorods across the sample volume. The scattered wave uniformly heats the cells. (B) The general setup of the laser rewarming application. NIR, near infrared.
Figure 6. Schematic diagram of the Joule heating setup
The left panel illustrates a configuration in which the sample is directly placed on a flat sheet electrical conductor. Upon application of power, Joule heating is initiated, with the bottom layer of the sample being heated first due to direct contact with the conductor. The right panel depicts a sandwich structure designed for tissue slices, enabling more uniform heating by enclosing the sample between conductive layers.
Figure 7. Molecular structure of water (left), DMSO (middle), and glycerol (right)
The red regions indicate areas of negative charge, while the blue regions represent positive charge. The black arrow denotes the direction of the electric field, illustrating the alignment behavior of dipolar molecules under an applied alternating electric field.
Figure 8. Configuration of capacitor heating system
(A) Concentric cylindrical electrode configuration; (B) Parallel plate electrode configuration; (C) Multiple electrode configuration. Each setup offers distinct electric field distributions and is suited for different sample sizes and heating uniformity requirements.
Figure 9. Schematic drawing of the antenna heating systems
The left panel illustrates a horn antenna system equipped with a microwave absorber. The microwave absorber is employed to prevent reflected waves from disrupting the electric field, and should be integrated within the horn antenna. The right panel depicts the hybrid helical antenna heating method combined with a water bath. The water bath is placed outside the sample holder to enhance heating efficiency.
Figure 10. SMER technology setup and results
(A)-(C) A schematic diagram of the SMER cavity with automatic loading and unloading system. (D) & (E) The simulation of the electric and magnetic field distribution. (F) The rewarming rate of the 25 mL DPVP solution from vitrified state to room temperature.
Figure 11. Eddy current illustration
The left figure shows a 3D representation of the eddy current generated by an alternating current-induced magnetic field. As the direction of the current changes, a magnetic flux is created and is located at the center of the coil. The right figure displays the top cross-section view. Eddy currents occur when the magnetic field is tangential to the surface of the conductive material.
Figure 12. Magnetic hysteresis effect and nanowarming results
(A) Illustration of the magnetic hysteresis curve (B-H). (B) Relationship between magnetic field strength and the specific absorption rate (SAR) at frequencies of 360 and 190 kHz, with power levels of 120 kW and 15 kW, respectively. SAR represents the rate at which energy is absorbed by biological tissue exposed to an electromagnetic field. (C) SAR of magnetic nanoparticles at various temperatures and magnetic field strengths.
Table 1. Water Bath Rewarming Experiments of Cryopreserved Samples
Sample Name Total Volume (mL) Water Bath Temperature(℃) Shaking Rewarming Rate (C/min) Recovery Rate (%) Rat Islet slice1[61] 0.1 37 - > 1000 79 Bovine embryo[25] 0.25 35 × > 1000 42.1 Testicular interstitial cell[62] 1 37 √ 20 65.3±2.1 Rabbit Carotid Artery[63] 10 37 × 130 71 DPVP Solution[48] 25 37 √ 48.2 - DPVP is a vitrification solution contains 41% (v/v) dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO) and 6% (w/v) polyvinylpyrrolidone (Sigma-Aldrich) in phosphate buffered saline solution (Sigma Aldrich). - means information is not mentioned in cited references. -
[1] Polge C, Smith A U, Parkes A S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature, 1949; 164(4172): 666. doi: 10.1038/164666a0 [2] Yánez-Ortiz I, Catalán J, Rodríguez-Gil J E, et al. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim Reprod Sci, 2022, 246: 106904 doi: 10.1016/j.anireprosci.2021.106904 [3] Bedaiwy M A, El-Nashar S A, El Saman A M, et al. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod, 2008; 23(12): 2709-2717. doi: 10.1093/humrep/den301 [4] Comizzoli P. Biobanking efforts and new advances in male fertility preservation for rare and endangered species. Asian J Androl, 2015; 17(4): 640-645. doi: 10.4103/1008-682X.153849 [5] Sharafi M, Borghei-Rad S M, Hezavehei M, et al. Cryopreservation of semen in domestic animals: a review of current challenges, applications, and prospective strategies. Animals-Basel, 2022; 12(23): 3271. doi: 10.3390/ani12233271 [6] Pence V C, Bruns E B. The tip of the iceberg: cryopreservation needs for meeting the challenge of exceptional plant conservation. Plants-Basel, 2022; 11(12): 1528. doi: 10.3390/plants11121528 [7] Rudick B, Opper N, Paulson R, et al. The status of oocyte cryopreservation in the united states. Fertil Steril, 2010; 94(7): 26422646. doi: 10.1016/j.fertnstert.2010.04.079 [8] Kim S S, Battaglia D E, Soules M R. The future of human ovarian cryopreservation and transplantation: fertility and beyond. Fertil Steril, 2001; 75(6): 1049-1056. doi: 10.1016/S0015-0282(01)01790-3 [9] Pomeroy K O, Comizzoli P, Rushing J S, et al. The art of cryopreservation and its changing landscape. Fertil Steril, 2022; 117(3): 469-476. doi: 10.1016/j.fertnstert.2022.01.018 [10] Wowk B. Thermodynamic aspects of vitrification. Cryobiology, 2010; 60(1): 11-22. doi: 10.1016/j.cryobiol.2009.05.007 [11] Huang Y, Dong Y, Gao B, et al. Transmembrane water transport and intracellular ice formation of human umbilical vein endothelial cells during freezing. Biopreserv Biobank, 2022; 20(4): 311-316. doi: 10.1089/bio.2022.0111 [12] Ma R, Peng J, Ren S, et al. Development of a 3-in-1 multifunctional cell processing system and optimization of cell type-dependent protocols for CPA addition/removal. Int Commun Heat Mass Transfer, 2025; 161: 108511. doi: 10.1016/j.icheatmasstransfer.2024.108511 [13] Hoffman D I, Zellman G L, Fair C C, et al. Cryopreserved embryos in the united states and their availability for research. Fertil Steril, 2003; 79(5): 1063-1069. doi: 10.1016/S0015-0282(03)00172-9 [14] Massip A, Vanderzwalmen P, Ectors F. Recent progress in cryopreservation of cattle embryos. Theriogenology, 1987; 27(1): 69-79. doi: 10.1016/0093-691X(87)90071-9 [15] Gao F, Ma R, Ren S, et al. Cryopreservation and biobanking of gametes, embryos, and reproductive tissues. Biopreserv Biobank, 2024; 22(1): 1-3. doi: 10.1089/bio.2024.29132.editorial [16] Taylor M J, Weegman B P, Baicu S C, et al. New approaches to cryopreservation of cells, tissues, and organs. Transfus Med Hemother, 2019; 46(3): 197-215. doi: 10.1159/000499453 [17] Ruiz-Delgado G J, Mancias-Guerra C, Tamez-Gomez E L, et al. Dimethyl sulfoxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol, 2009; 122(1): 1-5. doi: 10.1159/000227267 [18] Triana E, Ortega S, Azqueta C, et al. Thawing of cryopreserved hematopoietic progenitor cells from apheresis with a new dry-warming device. Transfusion, 2013; 53(1): 85-90. doi: 10.1111/j.1537-2995.2012.03669.x [19] Shu Z Q, Hughes S M, Fang C F, et al. A study of the osmotic characteristics, water permeability, and cryoprotectant permeability of human vaginal immune cells. Cryobiology, 2016; 72(2): 93-99. doi: 10.1016/j.cryobiol.2016.03.003 [20] Wang L, Fu R, Xu C, et al. Methods and applications of full-field optical coherence tomography: a review. J Biomed Opt, 2022; 27(5): 050901. doi: 10.1117/1.JBO.27.5.050901 [21] Mazur P, Leibo S P, Chu E H. A two-factor hypothesis of freezing injury. Evidence from chinese hamster tissue-culture cells. Exp Cell Res, 1972; 71(2): 345-355. doi: 10.1016/0014-4827(72)90303-5 [22] Gao D, Critser J K. Mechanisms of cryoinjury in living cells. ILAR J, 2000; 41(4): 187-196. doi: 10.1093/ilar.41.4.187 [23] Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol, 1984; 247(3 Pt 1): C125-142. doi: 10.1152/ajpcell.1984.247.3.C125 [24] Karlsson J O M, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials, 1996; 17(3): 243-256. doi: 10.1016/0142-9612(96)85562-1 [25] Hocki S, Semple E, Leibo S P. Effect of cooling and warming rates during cryopreservation on survival of in vitro-produced bovine embryos. Theriogenology, 1996; 46(5): 837-847. doi: 10.1016/S0093-691X(96)00241-5 [26] Bank H. Visualization of freezing damage. 2. Structural alterations during warming. Cryobiology, 1973; 10(2): 157-170. doi: 10.1016/0011-2240(73)90023-0 [27] Fahy G M M D R, Angell C A, et al. Vitrification as an approach to cryopreservation. Cryobiology, 1984; 21(4): 407-426. doi: 10.1016/0011-2240(84)90079-8 [28] Amorim C A, Curaba M, Van Langendonckt A, et al. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online, 2011; 23(2): 160-186. doi: 10.1016/j.rbmo.2011.04.005 [29] Shi Q, Xie Y, Wang Y, et al. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-analysis. Sci Rep, 2017; 7(1): 8538. doi: 10.1038/s41598-017-09005-7 [30] Sharma A, Lee C Y, Namsrai B E, et al. Cryopreservation of whole rat livers by vitrification and nanowarming. Ann Biomed Eng, 2023; 51(3): 566-577. doi: 10.1007/s10439-022-03064-2 [31] Solanki P K, Rabin Y. Thermomechanical stress analysis of rabbit kidney and human kidney during cryopreservation by vitrification with the application of radiofrequency heating. Cryobiology, 2021; 100: 180-192. doi: 10.1016/j.cryobiol.2021.01.002 [32] Zhao G, Liu Z F, Zhang A L, et al. Theoretical analyses of thermal stress of blood vessel during cryopreservation. Cryo Letters, 2005; 26(4): 239-250. [33] Solanki P K, Bischof J C, Rabin Y. Thermo-mechanical stress analysis of cryopreservation in cryobags and the potential benefit of nanowarming. Cryobiology, 2017; 76: 129-139. doi: 10.1016/j.cryobiol.2017.02.001 [34] Hua Z Z, Xu H Y, Zhou G Y, et al. Analyses of thermal stress and fracture during cryopreservation of blood vessel. Sci China Ser E-Technol Sci, 2000; 44: 158-163. doi: 10.1007/BF03014626 [35] Peng J, Ma R, Ren S, et al. A study of thermal stress generation during the rewarming process of cryopreserved large biomaterials. Cryobiology, 2021; 103: 167-168. doi: 10.1016/j.cryobiol.2021.11.042 [36] Yong K W, Laouar L, Elliott J A W, et al. Review of non-permeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology, 2020; 96: 1-11. doi: 10.1016/j.cryobiol.2020.08.012 [37] Bunnik E M. Ethics of allocation of donor organs. Curr Opin Organ Tran, 2023; 28(3): 192-196. doi: 10.1097/MOT.0000000000001058 [38] Panda K, Mazumder A, Krishnamurthy A. Kidneychain: leveraging blockchain & artificial intelligence for a streamlined organ donation solution. medRxiv, 2024. doi: 10.1101/2024.06.08.24308145 [39] Baust J G, Gao D, Baust J M. Cryopreservation: an emerging paradigm change. Organogenesis, 2009; 5(3): 90-96. doi: 10.4161/org.5.3.10021 [40] Giwa S, Lewis J K, Alvarez L, et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol, 2017; 35(6): 530542. doi: 10.1038/nbt.3889 [41] Olmo A, Barroso P, Barroso F, et al. The use of high-intensity focused ultrasound for the rewarming of cryopreserved biological material. IEEE Trans Ultrason Ferroelectr Freq Control, 2021; 68(3): 599607. doi: 10.1109/TUFFC.2020.3016950 [42] Xu R, Bradley E T, Eleanor M. Experiments and simulations demonstrating the rapid ultrasonic rewarming of frozen beef cryovials. arXiv preprint arXiv, 2022; 153(1): 517. doi: 10.1121/10.0016886 [43] Liu Y, Kangas J, Wang Y, et al. Photothermal conversion of gold nanoparticles for uniform pulsed laser warming of vitrified biomaterials. Nanoscale, 2020; 12(23): 12346-12356. doi: 10.1039/D0NR01614D [44] Zhan L, Guo S Z, Kangas J, et al. Conduction cooling and plasmonic heating dramatically increase droplet vitrification volumes for cell cryopreservation. Adv Sci(Weinh), 2021; 8(11): 2004605. doi: 10.1002/advs.202004605 [45] Alvarez C, Berrospe-Rodriguez C, Wu C, et al. Photothermal heating of titanium nitride nanomaterials for fast and uniform laser warming of cryopreserved biomaterials. Front Bioeng Biotechnol, 2022; 10: 957481. doi: 10.3389/fbioe.2022.957481 [46] Luo D, Yu C, He L, et al. Development of a single mode electromagnetic resonant cavity for rewarming of cryopreserved biomaterials. Cryobiology, 2006; 53(2): 288-293. doi: 10.1016/j.cryobiol.2006.07.001 [47] Pan J, Ren S, Sekar P K, et al. Investigation of electromagnetic resonance rewarming enhanced by magnetic nanoparticles for cryopreservation. Langmuir, 2019; 35(23): 7560-7570. doi: 10.1021/acs.langmuir.8b03060 [48] Ren S, Shu Z, Pan J, et al. Single-mode electromagnetic resonance rewarming for the cryopreservation of samples with large volumes: a numerical and experimental study. Biopreserv Biobank, 2022; 20(4): 317322. doi: 10.1089/bio.2022.0107 [49] Wang Z, Shu Z, Ren S, et al. Development of electromagnetic warming technology for cryopreservation. Annual Review of Heat Transfer, 2024; 27: 319-356. doi: 10.1615/AnnualRevHeatTransfer.2024055368 [50] Wang Z, Ren S, Shu Z, et al. An efficient and effective electromagnetic rewarming platform for cryopreservation. Cryobiology, 2024; 117: 104987. doi: 10.1016/j.cryobiol.2024.104987 [51] Etheridge M L, Xu Y, Rott L, et al. RF heating of magnetic nanoparticles improves the thawing of cryopreserved biomaterials. Technology, 2014; 2(3): 229-242. doi: 10.1142/S2339547814500204 [52] Manuchehrabi N, Gao Z, Zhang J, et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci Transl Med, 2017; 9(379): 4586. doi: 10.1126/scitranslmed.aah4586 [53] Sharma A, Rao J S, Han Z, et al. Vitrification and nanowarming of kidneys. Adv Sci(Weinh), 2021; 8(19): e2101691. doi: 10.1002/advs.202101691 [54] Rollig C, Babatz J, Wagner I, et al. Thawing of cryopreserved mobilized peripheral blood: comparison between waterbath and dry warming device. Cytotherapy, 2002; 4(6): 551-555. doi: 10.1080/146532402761624719 [55] Kilbride P, Meneghel J, Creasey G, et al. Automated dry thawing of cryopreserved haematopoietic cells is not adversely influenced by cryostorage time, patient age or gender. Plos One, 2020; 15(10): e0240310. doi: 10.1371/journal.pone.0240310 [56] Petrenko V, Whitworth R. Thermal properties of ice. Physics of ice. Oxford: Oxford University Press, 1999. [57] Marquet P. On the computation of moist-air specific thermal enthalpy. Q J Roy Meteor Soc, 2015; 141(686): 67-84. doi: 10.1002/qj.2335 [58] Melinder A. Thermophysical properties of aqueous solutions used as secondary working fluids. Stockholm: Royal Institute of Technology KTH, 2007. [59] Han H X, Zhan T J, Cui M D, et al. Investigation of rapid rewarming chips for cryopreservation by Joule heating. Langmuir, 2023; 39(31): 11048-11062. doi: 10.1021/acs.langmuir.3c01364 [60] Pegg D E. The history and principles of cryopreservation. Seminars in Reproductive Medicine, 2002; 20(3): 247-256. doi: 10.1055/s-2002-23515 [61] Bank H L, Davis R F, Emerson D. Cryogenic preservation of isolated rat islets of langerhans - effect of cooling and warming rates. Diabetologia, 1979; 16(3): 195-199. doi: 10.1007/BF01219798 [62] Gurina T M, Pakhomov A V, Polyakova A L, et al. The development of the cell cryopreservation protocol with controlled rate thawing. Cell Tissue Bank, 2016; 17(2): 303-316. doi: 10.1007/s10561-015-9533-6 [63] Song Y C, Pegg D E, Hunt C J. Cryopreservation of the common carotid artery of the rabbit: optimization of dimethyl sulfoxide concentration and cooling rate. Cryobiology, 2020; 93: 18-26. doi: 10.1016/j.cryobiol.2020.02.009 [64] Siu J Y, Liu C, Zhou Y. High-intensity focused ultrasound ablation around the tubing. PLoS One, 2017; 12(11): e0188206. doi: 10.1371/journal.pone.0188206 [65] Prakash P, Salgaonkar V A, Diederich C J. Modelling of endoluminal and interstitial ultrasound hyperthermia and thermal ablation: applications for device design, feedback control and treatment planning. Int J Hyperther, 2013; 29(4): 296-307. doi: 10.3109/02656736.2013.800998 [66] Zhou Y F. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol, 2011; 2(1): 8-27. doi: 10.5306/wjco.v2.i1.8 [67] Encabo L, Alcala E, Lopez-Soria J, et al. Hifu rewarming of organs after cold preservation: ex vivo assessment of heart performance in murine model. Transplantation, 2024; 108(1): E15-E7. doi: 10.1097/TP.0000000000004846 [68] Ziskin M C. Fundamental physics of ultrasound and its propagation in tissue. Radiographics, 1993; 13(3): 705-709. doi: 10.1148/radiographics.13.3.8316679 [69] Daly J, Zuchowicz N, Nunez Lendo C I, et al. Successful cryopreservation of coral larvae using vitrification and laser warming. Sci Rep, 2018; 8(1): 15714. doi: 10.1038/s41598-018-34035-0 [70] Khosla K, Zhan L, Bhati A, et al. Characterization of laser gold nanowarming: a platform for millimeter-scale cryopreservation. Langmuir, 2019; 35(23): 7364-7375. doi: 10.1021/acs.langmuir.8b03011 [71] Habibi M, Berger R D, Calkins H. Radiofrequency ablation: technological trends, challenges, and opportunities. EP Europace, 2021; 23(4): 511-519. doi: 10.1093/europace/euaa328 [72] Zheng H, Li P C, Ma R D, et al. Development of a three-dimensional multi-modal perfusion-thermal electrode system for complete tumor eradication. Cancers, 2022; 14(19): 4768. doi: 10.3390/cancers14194768 [73] Zhan L, Han Z H, Shao Q, et al. Rapid joule heating improves vitrification based cryopreservation. Nat Commun, 2022; 13(1): 6017. doi: 10.1038/s41467-022-33546-9 [74] Osepchuk J M. The history of the microwave oven: a critical review. IEEE, 2009. doi: 10.1109/MWSYM.2009.5165967 [75] Pegg D E, Green C J, Walter C A. Attempted canine renal cryopreservation using dimethyl sulphoxide helium perfusion and microwave thawing. Cryobiology, 1978; 15(6): 618-626. doi: 10.1016/0011-2240(78)90086-X [76] Lehr H B, Berggren R B, Summers A L, et al. Freezing and thawing of large organs. Cryobiology, 1964; 1(2): 194-197. doi: 10.1016/0011-2240(64)90011-2 [77] Wowk B, Phan J, Pagotan R, et al. 27 mhz constant field dielectric warming of kidneys cryopreserved by vitrification. Cryobiology, 2024; 115: 104893. doi: 10.1016/j.cryobiol.2024.104893 [78] Burdette E C, Karow A M, Jeske A H. Design, development, and performance of an electromagnetic illumination system for thawing cryopreserved kidneys of rabbits and dogs. Cryobiology, 1978; 15(2): 152-167. doi: 10.1016/0011-2240(78)90020-2 [79] Ruan H L, Wang T, Gao C. Microwave-water bath hybrid warming for frozen cryoprotectant solution using a helical antenna. Cryoletters, 2020; 41(1): 26-30. http://pubmed.ncbi.nlm.nih.gov/33973981/ [80] Rachman M J, Evans S, Pegg D E. Experimental results on the rewarming of a cryopreserved organ phantom in a uhf field. J Biomed Eng, 1992; 14(5): 397-403. doi: 10.1016/0141-5425(92)90085-Y [81] Ketterer F D, Holst H I, Lehr H B. Improved viability of kidneys with microwave thawing. Proc Cryobiol, 1971; 8(3): 309-315. doi: 10.1016/0011-2240(71)90197-0 [82] Guttman F M, Lizin J, Robitaille P, et al. Survival of canine kidneys after treatment with dimethyl-sulfoxide, freezing at --80 degrees c, and thawing by microwave illumination. Cryobiology, 1977; 14(5): 559-567. doi: 10.1016/0011-2240(77)90166-3 [83] Ma R, Ren S, Wang Z, et al. Electromagnetic rewarming for cryopreservation: a numerical comparison between multi-mode and single-mode electromagnetic cavity. Cryobiology, 2022; 109: 23. doi: 10.1016/j.cryobiol.2022.11.073 [84] Martin Paul Robinson D E P. Rapid electromagnetic warming of cells and tissues. IEEE Transactions on Biomedical Engineering, 1999; 46(10): 1175-1181. doi: 10.1109/10.804569 [85] Pan J, Shu Z, Ren S, et al. Determination of dielectric properties of cryoprotective agent solutions with a resonant cavity for the electromagnetic rewarming in cryopreservation. Biopreserv Biobank, 2017; 15(5): 404-409. doi: 10.1089/bio.2016.0096 [86] Ren S, Shu Z, Peng J, et al. Rapid and uniform rewarming by single-mode electromagnetic resonance cavity: effect of sample shape. Cryobiology, 2021; 103: 188. doi: 10.1016/j.cryobiol.2021.11.105 [87] Lewis J, Stoddart K, Reitinger V, et al. Molecular dynamics-informed optimization of cryoprotectant solutions for enhanced single-mode electromagnetic rewarming. Cryobiology, 2024; 117: 105091. doi: 10.1016/j.cryobiol.2024.105091 [88] Wang Z, Ren S, Shu Z, et al. Screening and optimization of cryoprotective agents(cpas) for electromagnetic heating of cryopreserved biomaterials. Cryobiology, 2022; 109: 16. doi: 10.1016/j.cryobiol.2022.11.052 [89] Ren S, Shu Z, Wang Z, et al. Successful vitreous cryopreservation of rabbit jugular vein using magnetic nanoparticles enhanced single-mode electromagnetic resonance rewarming system. Cryobiology, 2021; 103: 173-174. doi: 10.1016/j.cryobiol.2021.11.060 [90] Wang W. Design of low-noise low-power ecg amplifier circuit with high integration level. Journal of Physics Conference Series, 2023; 2649(1): 012062. doi: 10.1088/1742-6596/2649/1/012062 [91] Sun J, Wang W, Yue Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials(Basel), 2016; 9(4): 231. doi: 10.3390/ma9040231 [92] Han Z H, Rao J S, Gangwar L, et al. Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model. Nat Commun, 2023; 14(1): 3407. doi: 10.1038/s41467-023-38824-8 [93] Gangwar L, Han Z, Scheithauer C, et al. Physical vitrification and nanowarming at human organ scale to enable cryopreservation. bioRxiv, 2024. doi: 10.1101/2024.11.08.622572 [94] Bischof J C, Oziri O J, Rao J S, et al. Scalable purification of iron oxide nanoparticles by tangential flow filtration for organ cryopreservation and transplantation. SPIE, 2025. doi: 10.1117/12.3043417 [95] Ye Z, Tai Y, Han Z, et al. Engineering magnetic nanoclusters for highly efficient heating in radio-frequency nanowarming. Nano Lett, 2024; 24(15): 4588-4594. doi: 10.1021/acs.nanolett.4c00721 [96] Han Z, Gangwar L, Namsrai B-E, et al. Kidney tissue loading reduces the critical cooling and warming rates of vs55 and vmp cryoprotective solutions. Cryobiology, 2024; 117: 104977. doi: 10.1016/j.cryobiol.2024.104977 -


 投稿系统
投稿系统


 下载:
下载: