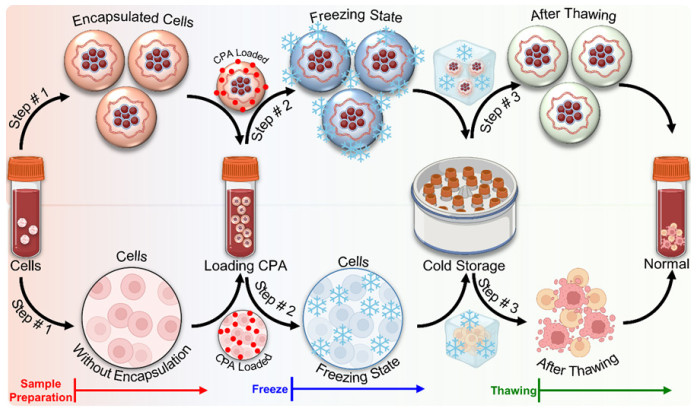
-
Abstract: Cryopreservation is a fundamental technology in biomedical research, regenerative medicine, and tissue engineering, enabling the long-term storage of cells, tissues, and organs. However, its effectiveness is limited by challenges such as intracellular ice formation, cryoprotectant toxicity, and reduced post-thaw viability. This review explores the crucial role of encapsulation in enhancing cryopreservation efficiency, with a focus on recent advances in materials science, bioengineering, and cryobiology. Emerging technologies, such as nanotechnology and stimuli-responsive polymers, are transforming encapsulation strategies. Innovations such as microfluidic systems offer precise control over cooling rates and cryoprotectant distribution, thereby mitigating conventional limitations. The review also addresses current obstacles related to scaling up encapsulation processes and ensuring the long-term biocompatibility and stability of preserved specimens. By synthesizing recent findings, this work provides a comprehensive resource for researchers and clinicians seeking to enhance biopreservation techniques and their applications in contemporary medicine and biotechnology. Finally, the review identifies critical knowledge gaps that must be addressed to improve the efficacy of cryopreservation strategies and advance their clinical translation.
-
Key words:
- cryopreservation /
- encapsulation /
- hydrogels /
- biomaterials /
- tissue engineering /
- regenerative medicine /
- nanotechnology /
- smart polymers
-
Figure 2. Overview of encapsulation techniques for cryopreservation
(A) Schematic of a capillary microfluidics-based core-shell device, in which cells are encapsulated in droplets using a tube-in-tube system and cross-linked in CaCl2 solution to form stable microcapsules. (B) Illustration of stem cell encapsulation and vitrification using quartz microcapillaries and plastic straws, including cryomicroscopy to monitor ice formation inhibition. Insets show alginate hydrogel microdroplets and stem cell-laden capsules. (C) Diagram of an electrostatic spraying system used to generate core-shell microcapsules and monitor vitrification in a water bath. (D) Schematic of a centrifugal platform used to fabricate core-shell structures and hydrogel beads/fibers. (E) Characterization of microcapsule formation as a function of oil flow rate, demonstrating control over microcapsule diameter and shell thickness. (F) Fluorescence microscopy images and size distribution of electrostatically sprayed microcapsules. (G) Microscopic evaluation of encapsulation efficiency for murine embryonic stem cells and human adipose-derived stem cells. (H) Morphological characterization and size distribution of hydrogel fibers and capsules generated via centrifugal techniques. (I) Comparison of perivascular Adipose-Derived Stem Cell (pADSC) viability among fresh, CPA-treated, and cryopreserved samples. (J) Viability assessment of encapsulated versus non-encapsulated stem cells before and after vitrification. (K) Cell viability comparison between conventional slow freezing and core-shell encapsulation methods for HUVECs. (L) Short-term viability analysis of cells encapsulated in simple hydrogel beads/fibers versus core-shell microcapsules. All panels reproduced with permission: Figure (A, E, I)[40], (B, G, J)[41], (C, F, K)[37], (D, H, L)[42]. * indicates P < 0.05, ** indicates P < 0.01.
-
[1] Moniz I, Soares M, Sousa A P, et al. The low survivability of transplanted gonadal grafts: the impact of cryopreservation and transplantation conditions on mitochondrial function. Biology (Basel), 2024; 13(7): 542. doi: 10.3390/biology13070542 [2] Chang T, Zhao G. Ice inhibition for cryopreservation: materials, strategies, and challenges. Adv Sci (Weinh), 2021; 8(6): 2002425. doi: 10.1002/advs.202002425 [3] Bojic S, Murray A, Bentley B L, et al. Winter is coming: the future of cryopreservation. BMC Biol, 2021; 19(1): 56. doi: 10.1186/s12915-021-00976-8 [4] Pegg D E. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology, 2020; 93: 3-11. doi: 10.1016/j.cryobiol.2020.01.005 [5] Netshirovha T R, Makumbane V, Sehlabela L D, et al. Cryopreservation of oocyte in livestock animals: principles, techniques and updated outcomes. 2024. [6] von Bomhard A, Elsässer A, Ritschl L M, et al. Cryopreservation of endothelial cells in various cryoprotective agents and media - vitrification versus slow freezing methods. PLoS One, 2016; 11(2): e0149660. doi: 10.1371/journal.pone.0149660 [7] Rajan R, Matsumura K. Development and application of cryoprotectants. Survival strategies in extreme cold and desiccation: Adaptation mechanisms and their applications. Adv Exp Med Bio, 2018; 1081: 339-354. doi: 10.1007/978-981-13-1244-1_18 [8] Zhang C, Zhou Y, Zhang L, et al. Hydrogel cryopreservation system: an effective method for cell storage. Int J Mol Sci, 2018; 19(11): 3330. doi: 10.3390/ijms19113330 [9] Marcantonini G, Bartolini D, Zatini L, et al. Natural cryoprotective and cytoprotective agents in cryopreservation: a focus on melatonin. Molecules, 2022; 27(10): 3254. doi: 10.3390/molecules27103254 [10] Naranjo-Alcazar R, Bendix S, Groth T, et al. Research progress in enzymatically cross-linked hydrogels as injectable systems for bioprinting and tissue engineering. Gels, 2023; 9(3): 230. doi: 10.3390/gels9030230 [11] Hu W, Wang Z, Xiao Y, et al. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci, 2019; 7(3): 843-855. doi: 10.1039/C8BM01246F [12] Wang X, Wang E, Zhao G. Advanced cryopreservation engineering strategies: the critical step to utilize stem cell products. Cell Regen, 2023; 12(1): 28. doi: 10.1186/s13619-023-00173-8 [13] Yan X, Chen Y R, Song Y F, et al. Advances in the application of supramolecular hydrogels for stem cell delivery and cartilage tissue engineering. Front Bioeng Biotechnol, 2020; 8: 847. doi: 10.3389/fbioe.2020.00847 [14] Tianjin Da Xue. Transactions of Tianjin University. Tianjin: The University, 2005: Vol. 11. [15] Aabling R R, Alstrup T, Kjær E M, et al. Reconstitution and post-thaw storage of cryopreserved human mesenchymal stromal cells: pitfalls and optimizations for clinically compatible formulants. Regen Ther, 2023; 23: 67-75. doi: 10.1016/j.reth.2023.03.006 [16] Watanabe T, Okitsu T, Ozawa F, et al. Millimeter-thick xenoislet-laden fibers as retrievable transplants mitigate foreign body reactions for longterm glycemic control in diabetic mice. Biomaterials, 2020; 255: 120162. doi: 10.1016/j.biomaterials.2020.120162 [17] Benson E E, Harding K, Ryan M, et al. Alginate encapsulation to enhance biopreservation scope and success: a multidisciplinary review of current ideas and applications in cryopreservation and non-freezing storage. Cryoletters, 2018; 39(1): 14-38. http://pubmed.ncbi.nlm.nih.gov/29734412/ [18] Han Y, Sun M, Lu X, et al. A 3D printable gelatin methacryloyl/chitosan hydrogel assembled with conductive PEDOT for neural tissue engineering. Composites Part B: Engineering, 2024; 273: 111241. doi: 10.1016/j.compositesb.2024.111241 [19] Guan S, Wang Y, Xie F, et al. Carboxymethyl chitosan and gelatin hydrogel scaffolds incorporated with conductive PEDOT nanoparticles for improved neural stem cell proliferation and neuronal differentiation. Molecules, 2022; 27(23): 8326. doi: 10.3390/molecules27238326 [20] Todros S, Spadoni S, Barbon S, et al. Compressive mechanical behavior of partially oxidized polyvinyl alcohol hydrogels for cartilage tissue repair. Bioengineering, 2022; 9(12): 789. doi: 10.3390/bioengineering9120789 [21] Liu Y, Zhang D, Tang Y, et al. Development of a radical polymerization algorithm for molecular dynamics simulations of antifreezing hydrogels with double-network structures. NPJ Comput Mater, 2023; 9(1): 209. doi: 10.1038/s41524-023-01161-x [22] Bercea M. Recent advances in poly (vinyl alcohol)-based hydrogels. Polymers, 2024; 16(14): 2021. doi: 10.3390/polym16142021 [23] Tenório-Neto E T. Poly (ethylene glycol)-based hydrogels from preparation methods to applications. Journal of Colloid Science and Biotechnology, 2016; 5(1): 2-15. doi: 10.1166/jcsb.2016.1139 [24] Li C, Qian Y, Zhao S, et al. Alginate/PEG based microcarriers with cleavable crosslinkage for expansion and non-invasive harvest of human umbilical cord blood mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl, 2016; 64: 43-53. doi: 10.1016/j.msec.2016.03.089 [25] Underwood L A. Development and optimization of a preservable three-dimensional bio-construct. Michigan: University of Michigan-Dearborn, 2022. [26] Mahou R, Wandrey C. Alginate-Poly (ethylene glycol) hybrid microspheres with adjustable physical properties. Macromolecules, 2010; 43(3): 1371-1378. doi: 10.1021/ma902469f [27] Gok O. Physicochemical effects of PEG content in alginate-based double network hydrogels as hybrid scaffolds. TJST, 2024; 19(1): 249256. doi: 10.55525/tjst.1410187 [28] Nakamura M, Matsumoto M, Ito T, et al. Microfluidic device for the high-throughput and selective encapsulation of single target cells. Lab Chip, 2024; 24(11): 2958-2967. doi: 10.1039/D4LC00037D [29] Ling S D, Geng Y, Chen A, et al. Enhanced single-cell encapsulation in microfluidic devices: From droplet generation to single-cell analysis. Biomicrofluidics, 2020; 14(6): 061508. doi: 10.1063/5.0018785 [30] Kumar A, Brown R A, Roufaeil D B, et al. DeepFreeze 3D-biofabrication for bioengineering and storage of stem cells in thick and large-scale human tissue analogs. Adv Sci (Weinh), 2024; 11(11): e2306683. doi: 10.1002/advs.202306683 [31] Zhan Y, Jiang W, Liu Z, et al. Utilizing bioprinting to engineer spatially organized tissues from the bottom-up. Stem Cell Res Ther, 2024; 15(1): 101. doi: 10.1186/s13287-024-03712-5 [32] Li J, Moeinzadeh S, Kim C, et al. Development and systematic characterization of GelMA/alginate/PEGDMA/xanthan gum hydrogel bioink system for extrusion bioprinting. Biomaterials, 2023; 293: 121969. doi: 10.1016/j.biomaterials.2022.121969 [33] Warburton L, Rubinsky B. Cryopreservation of 3D bioprinted scaffolds with temperature-controlled-cryoprinting. Gels, 2023; 9(6): 502. doi: 10.3390/gels9060502 [34] Borges J, Zeng J, Liu X Q, et al. Recent developments in layer-by-layer assembly for drug delivery and tissue engineering applications. Adv Healthc Mater, 2024; 13(8): e2302713. doi: 10.1002/adhm.202302713 [35] Su R, Wang F, McAlpine M C. 3D printed microfluidics: advances in strategies, integration, and applications. Lab Chip, 2023; 23(5): 12791299. doi: 10.1039/D2LC01177H [36] Dou M, Lu C, Rao W. Bioinspired materials and technology for advanced cryopreservation. Trends Biotechnol, 2022; 40(1): 93-106. doi: 10.1016/j.tibtech.2021.06.004 [37] Li Y, Memon K, Zheng Y, et al. Microencapsulation facilitates lowcryoprotectant vitrification of human umbilical vein endothelial cells. ACS Biomater Sci Eng, 2019; 5(10): 5273-5283. doi: 10.1021/acsbiomaterials.9b00726 [38] Meneghel J, Kilbride P, Morris G J. Cryopreservation as a key element in the successful delivery of cell-based therapies-a review. Front Med (Lausanne), 2020; 7: 592242. doi: 10.3389/fmed.2020.592242 [39] Kavand A, Noverraz F, Gerber-Lemaire S. Recent advances in alginate-based hydrogels for cell transplantation applications. Pharmaceutics, 2024; 16(4): 469. doi: 10.3390/pharmaceutics16040469 [40] Zhao G, Liu X, Zhu K, et al. Hydrogel encapsulation facilitates rapidcooling cryopreservation of stem cell-laden core-shell microcapsules as cell-biomaterial constructs. Adv Healthc Mater, 2017; 6(23): 10. doi: 10.1002/adhm.201700988 [41] Huang H, Choi J K, Rao W, et al. A lginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-CPA cell vitrification. Adv Funct Mater, 2015; 25(44): 6939-6850. doi: 10.1002/adfm.201503047 [42] Cheng Y, Zhang X Z, Cao Y, et al. Centrifugal microfluidics for ultrarapid fabrication of versatile hydrogel microcarriers. Applied Materials Today, 2018; 13: 116-125. doi: 10.1016/j.apmt.2018.08.012 -


 投稿系统
投稿系统


 下载:
下载: