Metabolomics study on dibenz[a, h]anthracene exposure-induced pulmonary injury in rats after intratracheal instillation
doi: 10.1515/fzm-2025-0004
-
Abstract:
Background Northern residents predominantly rely on coal-fired heating during winter, leading to severe air pollution. Polycyclic aromatic hydrocarbons (PAHs) adsorbed on atmospheric particulate matter pose significant health risks. Among PAHs, dibenz[a, h]anthracene (DahA), though present at lower environmental concentrations compared to other PAHs, exhibits a carcinogenic potency that is 10 or more times greater than benzo[a]pyrene (BaP), underscoring its potential harm. Despite reports on DahA's multiple toxic effects, its impact on metabolic networks remains poorly understood. Methods Based on the respiratory volume of adult rats and the concentration of PM2.5-bound DahA in heavily polluted cities of northern China, adult Sprague-Dawley rats were treated with DahA (0.07 μg/kg and 0.2 μg/kg) twice weekly for four weeks via intratracheal instillation. Metabolomic profiling of serum was performed using rapid resolution liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (RRLC/Q-TOF-MS) to elucidate metabolic disruptions caused by DahA exposure. Results DahA exposure induced significant oxidative stress and inflammatory responses in rats, accompanied by notable alterations in the serum metabolome. A total of 11 metabolites were found to be decreased, and 2 metabolites were increased, with disruptions observed in folate biosynthesis, glycerophospholipid metabolism, and nitrogen metabolism pathways. Additionally, metabolic dysregulation may interfere with the tricarboxylic acid cycle and compromise nucleotide homeostasis. Conclusion These findings enhance our understanding of the toxicological effects of DahA exposure and its role in lung damage. The results suggest that metabolic disturbances caused by DahA may contribute to the exacerbation of respiratory diseases associated with particulate matter-bound PAH pollution during the heating season in cold regions. -
Key words:
- DahA /
- metabolomics /
- pulmonary injury /
- heating season
-
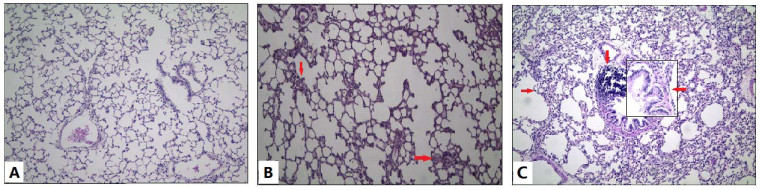
Figure 1. Photomicrographs of rat lungs (HE staining)
(A) Control group: Lung structures appeared nearly normal with only slight inflammation; (B) DL group: Lung structures remained nearly normal, but inflammatory cell infiltration was more pronounced compared to the control group, as indicated by the arrows; (C) DH group: Compared with the control group, thickened alveolar walls and proliferation of fibrous tissue were observed, as indicated by the arrows in the exposed groups. Original magnification: ×200; small window in exposed group: ×400.
Figure 2. Score plot with PLS-DA and permutation test of serum metabolites in control (CON) and exposure groups
Panel (A) shows the score plot derived from PLS-DA analysis, while panel (B) presents the results of the permutation test, comparing the serum metabolite profiles between the CON and exposure groups.
Figure 3. Trends in alterations of different metabolites in serum comparing DahA exposed groups with the control group
The horizontal axis represents the ratio of the metabolite in the exposure group compared with the control group, calculated as (Cexposed-Ccontrol) / Ccontrol. This plot illustrates the changes in metabolite levels following DahA exposure.
Figure 4. Pathway analysis of biomarkers using MetaboAnalyst 3.0
(1) Folate biosynthesis; (2) Glycerophospholipid metabolism; (3) Nitrogen me-tabolism; (4) Sphingolipid metabolism. The analysis highlights key metabolic pathways impacted by DahA exposure, identifying potential biomarkers associ-ated with pulmonary toxicity.
Figure 5. Toxic effects of metabolic changes induced by DahA exposure
The serum metabolomic changes reveal that exposure to DahA disrupts key metabolic pathways, including folate biosynthesis, lipid metabolism, nitrogen metabolism, and amino acid metabolism. These disruptions contribute to heightened inflammatory responses and oxidative stress in the lungs, providing insight into the mechanisms underlying DahA-induced pulmonary toxicity.
Table 1. The fundamental variables between the control group and the DahA exposure group during the experiment
Control (N = 8) DL(N=8) DH(N=8) Body weight (g) 304.5 ± 15.7 298.4 ± 18.8 296.0 ± 23.4 Cytotoxicity and inflammation cytokine in BALF LDH (U/L) 121.51 ± 25.92 165.55 ± 70.02* 180.06 ± 61.27* AKP (U/100mL) 3.48 ± 1.27 4.16 ± 1.18 7.08 ± 2.10* TP (mg/L) 158.27 ± 59.60 190.47 ± 85.81* 242.05 ± 57.68* IL-6 (pg/mL) 101.06 ± 28.57 126.15 ± 73.21 154.10 ± 42.51* TNF-α (pg/mL) 28.15 ±8.47 45.12 ±10.59* 40.71 ±18.81 Inflammatory and oxidant stress cytokine levels in serum SOD (mg/mL) 32.59 ±3.65 21.74 ±4.67* 17.55 ±3.94* MDA (mg/mL) 1.03 ± 0.30 1.17 ± 0.38 1.27 ± 0.38 IL-6 (pg/mL) 32.68 ±14.52 47.27 ±15.37 53.88 ±18.66* TNF-α (pg/mL) 22.14 ±9.28 26.07 ±11.34 27.46 ±8.85 Markers of oxidative stress in rat lung tissue SOD (U/mg prot) 82.72 ±18.84 60.53 ±17.49* 55.47 ±19.95* MDA (nmol/mg prot) 1.51 ± 0.52 2.47 ± 0.55* 2.03 ± 0.46 i-NOS (U/mg prot) 0.58 ± 0.31 0.83 ± 0.32 1.21 ± 0.76* e-NOS (U/mg prot) 2.13 ± 1.06 1.58 ± 0.85 1.37 ± 0.45 NO (nmol/mg prot) 1.71 ± 0.41 2.37 ± 0.53 2.91 ± 0.67* DL: low-dose DahA-exposed; DH: high-dose DahA-exposed; BALF: bronchoalveolar lavage fluid; LDH: lactate dehydrogenase; AKP: alkaline phosphatase; TP: total protein; IL: Interleukin; TNF: tumor necrosis factor; SOD: Superoxide dismutase; MDA: malondialdehyde; NOS: nitric oxide synthase; NO: nitric oxide. *P value < 0.05 was considered significantly compared with the control group. Table 2. Important metabolites in RRLC/Q-TOF-MS positive ion modes (ESI+)
NO. VIP RT Actual mass Exact mass Mass error (ppm) Molecule composition Idetity Adduct Pathway 1 1.934 17.819 780.543 780.554 14.374 C44H78NO8P PE-NMe (18:1/20:4) M+H Glycerophospholipid metabolism 2 1.854 17.999 104.106 104.108 11.371 C5H14NO Choline M+H Gly, serine and threonine metabolism 3 1.835 18.452 205.086 205.082 -16.249 C7H12N2O5 Glutamylglycine M+H Gly, serine and threonine metabolism 4 1.832 27.710 141.990 141.991 1.562 CH4NO5P Carbamoyl phosphate M+H Nitrogen metabolism 5 1.826 15.024 444.169 444.163 -13.058 C19H21N7O6 Dihydrofolic acid M+H Folate biosynthesis 6 1.800 20.461 551.376 551.371 -8.524 C28H55O8P PA (i-13:0/i-12:0) M+H Glycerophospholipid metabolism 7 1.788 22.563 806.556 806.545 -13.252 C42H79NO11S 3-O-Sulfogalactosylceramide M+H Sphingolipid metabolism 8 1.787 18.439 302.144 302.151 21.161 C16H19N3O3 Prolyl-Tryptophan M+H Tryptophan metabolism 9 1.734 21.066 329.244 329.247 9.112 C22H32O2 Docosahexaenoic acid M+H Unsaturated fatty acid metabolism 10 1.675 24.426 761.642 761.654 15.602 C43H89N2O6P SM (d18:0/20:0) M+H Sphingolipid metabolism 11 1.654 16.147 568.335 568.340 9.015 C30H50NO7P LysoPC (22:6) M+H Lysophospholipid metabolism 12 1.638 18.365 510.353 510.356 5.878 C25H52NO7P LysoPE (0:0/20:0) M+H Lysophospholipid metabolism 13 1.615 11.930 261.145 261.145 0 C11H20N2O5 Isoleucyl-Glutamate M+H Nitrogen metabolism PE: phosphatidylethanolamine; PA: phosphatidic acid; SM: sphingomyelin. -
[1] Patel A B, Shaikh S, Jain K R, et al. Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol, 2020; 11, 562813. doi: 10.3389/fmicb.2020.562813 [2] Hertz-Picciotto I, Baker R J, Yap P S, et al. Early childhood lower 48 respiratory illness and air pollution. Environ Health Perspect, 2007; 115(10): 1510-1518. doi: 10.1289/ehp.9617 [3] Jeng H A, Pan C H, Diawara N, et al. Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup Environ Med, 2011; 68(9): 653-658. doi: 10.1136/oem.2010.055020 [4] Perera F P, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics, 2009; 124(2): e195-e202. doi: 10.1542/peds.2008-3506 [5] Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol, 2011; 31(3): 363-373. doi: 10.1016/j.reprotox.2010.12.055 [6] Wassenberg D M, Di Giulio R T. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect, 2004; 112(17): 1658-1664. doi: 10.1289/ehp.7168 [7] Kim A, Park M, Yoon T K, et al. Maternal exposure to benzo[b] fluoranthene disturbs reproductive performance in male offspring mice. Toxicol Lett, 2011; 203(1): 54-61. doi: 10.1016/j.toxlet.2011.03.003 [8] Kummer V, Mašková J, Matiašovic J, et al. Morphological and functional disorders of the immature rat uterus after postnatal exposure to benz[a]anthracene and benzo[k]fluoranthene. Environ Toxicol Pharmacol, 2009; 27(2): 253-258. doi: 10.1016/j.etap.2008.11.001 [9] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum, 2010; 92: 1-853. [10] Kang Z, Liu, X B, Yang, C, et al. Effect of ambient PM2.5-bound BbFA and DahA on small airway dysfunction of primary schoolchildren in northeast China. Biomed Res Int, 2019; 2019: 2457964. doi: 10.1155/2019/2457964 [11] Malik A I, Rowan-Carroll A, Williams A, et al. Hepatic genotoxicity and toxicogenomic responses in Muta™Mouse males treated with dibenz[a, h]anthracene. Mutagenesis, 2013; 28(5): 543-554. doi: 10.1093/mutage/get031 [12] Okona-Mensah K B, Battershill J, Boobis A, et al. An approach to investigating the importance of high potency polycyclic aromatic hydrocarbons (PAHs) in the induction of lung cancer by air pollution. Food Chem Toxicol, 2005; 43(7): 1103-1116. doi: 10.1016/j.fct.2005.03.001 [13] Wang X F, Jiang S F, Liu Y, et al. Comprehensive pulmonary metabolome responses to intratracheal instillation of airborne fine particulate matter in rats. Sci Total Environ, 2017; 592: 41-50. doi: 10.1016/j.scitotenv.2017.03.064 [14] Xu Y Y, Wang W J, Zhou J, et al. Metabolomics analysis of a mouse model for chronic exposure to ambient PM2.5. Environ Pollut, 2019; 247: 953-963. doi: 10.1016/j.envpol.2019.01.118 [15] Zhang Y N, Li Y B, Shi Z X, et al. Metabolic impact induced by total, water soluble and insoluble components of PM acute exposure in mice. Chemosphere, 2018; 207: 337-346. doi: 10.1016/j.chemosphere.2018.05.098 [16] He M, Ichinose T, Yoshida S, et al. PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol, 2017; 37(10): 1203-1218. doi: 10.1002/jat.3482 [17] Li R J, Kou X J, Xie L Z, et al. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ Sci Pollut Res Int, 2015; 22(24): 20167-20176. doi: 10.1007/s11356-015-5222-z [18] Roberts L D, Souza A L, Gerszten R E, et al. Targeted metabolomics. Curr Protoc Mol Biol, 2012; Chapter 30, Unit 30.2-30.2.24. [19] Sun M, Gao X Q, Zhang D W, et al. Identification of biomarkers for unstable angina by plasma metabolomic profiling. Mol Biosyst, 2013; 9(12): 3059-3067. doi: 10.1039/c3mb70216b [20] Snyder S H, Bredt D S. Biological roles of nitric oxide. Sci Am, 1992; 266(5): 68-71, 74-77. doi: 10.1038/scientificamerican0592-68 [21] Virág L, Szabó E, Gergely P, et al. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett, 2003; 11: 113-124, 140-141. doi: 10.1016/S0378-4274(02)00508-8 [22] Zhao Y Y, Wang H L, Cheng X L, et al. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci Rep, 2015; 5: 12936. doi: 10.1038/srep12936 [23] Walker A K, Jacobs R L, Watts J L, at al. A conserved SREBP-1/ phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell, 2011; 147(4): 840-852. doi: 10.1016/j.cell.2011.09.045 [24] Hite R D, Seeds M C, Safta A M, et al. Lysophospholipid generation and phosphatidylglycerol depletion in phospholipase A(2)-mediated surfactant dysfunction. Am J Physiol Lung Cell Mol Physiol, 2005; 288(4): L618-L624. doi: 10.1152/ajplung.00274.2004 [25] Hu W Y, Dong T Y, Wang L L, et al. Obesity aggravates toxic effect of BPA on spermatogenesis. Environ Int, 2017; 105: 56-65. doi: 10.1016/j.envint.2017.04.014 [26] Sun H, Zhao J, Zhong D, et al. Potential serum biomarkers and metabonomic profiling of serum in ischemic stroke patients using UPLC/Q-TOF MS/MS. PloS one, 2017; 12(12): e0189009. doi: 10.1371/journal.pone.0189009 [27] Ebenezer D L, Fu P, Natarajan V. Targeting sphingosine-1-phosphate signaling in lung diseases. Pharmacol Ther, 2016; 168: 143-157. doi: 10.1016/j.pharmthera.2016.09.008 [28] Iwatsuki K, Torii K. Peripheral chemosensing system for tastants and nutrients. Curr Opin Endocrinol Diabetes Obes, 2012; 19(1): 19-25. doi: 10.1097/MED.0b013e32834ec7f8 [29] Salbaum J M, Kappen C. Genetic and epigenomic footprints of folate. Prog Mol Biol Transl Sci, 2012; 108: 129-158. doi: 10.1016/B978-0-12-398397-8.00006-X [30] Kim J, Hu Z, Cai L, et al. CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells. Nature, 2017; 546(7656): 168-172. doi: 10.1038/nature22359 [31] Pausch J, Rasenack J, Häussinger D, et al. Hepatic carbamoyl phosphate metabolism. Role of cytosolic and mitochondrial carbamoyl phosphate in de novo pyrimidine synthesis. Eur J Biochem, 1985; 150(1): 189-194. doi: 10.1111/j.1432-1033.1985.tb09006.x [32] Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med, 2009; 30(1-2): 29-41. doi: 10.1016/j.mam.2008.08.003 [33] Ni H, Lu L, Deng J, et al. Effects of glutamate and aspartate on serum antioxidative enzyme, sex hormones, and genital inflammation in boars challenged with hydrogen peroxide. Mediators Inflamm, 2016; 2016: 4394695. doi: 10.1155/2016/4394695 [34] Waclawiková B, El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals, 2018; 11(3): 63. doi: 10.3390/ph11030063 -


 投稿系统
投稿系统


 下载:
下载: