Small ubiquitin-like modifiers inhibitors lower blood pressure via ERK5/KLF2-dependent upregulation of the eNOS/NO pathway
doi: 10.1515/fzm-2024-0020
-
Abstract:
Background Small ubiquitin-like modifiers (SUMO)ylation is a dynamic and reversible post-translational modification playing pivotal roles in the regulation of cancer, diabetes, heart failure, and neurological diseases. However, whether SUMO inhibitors also have anti-hypertension effect remains yet to be explored. Methods Blood pressure was monitored in spontaneously hypertensive rats (SHR) after Tannic acid (TA) administration for 4 weeks. The contents of nitric oxide (NO) and endothelin-1 (ET-1) in the serum of SHR were measured. Isolated endothelium-intact mesenteric artery rings were used to study relaxation effect of SUMO inhibitors. ERK5 SUMOylation was determined using coimmunoprecipitation (co-IP) and immunofluorescence (IF). NO levels were analyzed by IF. The expression levels of KLF2 and p-eNOS were semiquantified by Western blot analysis. The transcriptional activity of eNOS promotor was assayed using ChIP-PCR. Results Three SUMO inhibitors all reduced the phenylephrine (PE)-induced contraction of mesenteric artery rings in a concentration-dependent manner. Co-IP revealed that ponatinib promoted ERK5 SUMOylation, which was nulled following pretreatment with the SUMO inhibitors. IF displayed that TA increased ERK5 accumulation and its co-localization with SUMO-1 in the nucleus. ChIP-PCR unveiled TA-induced enhancement of KLF2-dependent eNOS promoter activity and upregulation of eNOS/NO expression in HUVECs. In vivo, TA significantly lowered the blood pressure and improved the vascular reactivity by activating the KLF2/eNOS/NO pathway. Additionally, the level of NO was elevated along with decreased ET-1 levels in the serum of SHR. Conclusions SUMO inhibitors inhibit ERK5 SUMOylation to promote KLF2-eNOS/NO signaling, indicating their therapeutic potential for the treatment of hypertension. -
Key words:
- hypertension /
- SUMO inhibitor /
- ERK5 /
- SUMOylation /
- KLF2
-
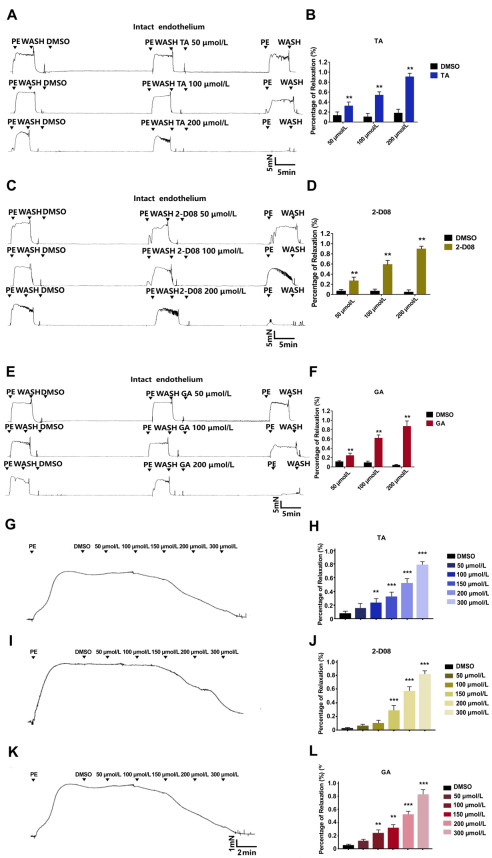
Figure 1. SUMO inhibitors exert vasorelaxant effect on phenylephrine (PE)-preconstricted endothelium-intact mesenteric artery rings in a dose-dependent manner
The effects of Tannic acid (TA) at different concentrations on PE-preconstricted mesenteric artery rings were measured by (A) the typical recording and (B) bar chart on statistical data. The effects of 2-D08 at different concentrations on PE-preconstricted mesenteric artery rings were measured by (C) the typical recording and (D) statistical data. The effects of Ginkgolic acid (GA) at different concentrations on PE-preconstricted mesenteric artery rings were measured by (E) the typical recording and (F) statistical data. The typical recording (G) and relative bar graph (H) showed PE-induced contraction in isolated artery after acute stimulation with TA for 2 min. The typical recording (I) and statistical data (J) showed PE-induced contraction in isolated artery after acute stimulation with 2-D08 for 2 min. The typical recording (K) and statistical data (L) showed PE-induced contraction in isolated artery after acute stimulation with GA for 2 min. N = 6. Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001, compared to control.
Figure 2. Ponatinib eliminates the vasorelaxant effect of SUMO inhibitors on vasorelaxation of phenylephrine (PE)-preconstricted endothelium-intact mesenteric artery rings via extracellular signal-regulated kinase 5 (ERK5) SUMOylation
The interaction between ERK5 and SUMO was detected by co-immunoprecipitation (Co-IP) in HUVECs after incubation with (A) Tannic acid (TA), (B) 2-D08, and (C) Ginkgolic acid (GA) with or without ponatinib (200 nmol/L) for 24 h. N = 3. The contraction-responses of TA with pre-treatment of ponatinib (200 nmol/L) for 20 min to PE-induced constriction by (D) the typical recording and (E) bar chart on statistical data. The contraction-responses of 2-D08 with pre-treatment of ponatinib (200 nmol/L) for 20 min to PE-induced constriction by (F) the typical recording and (G) statistical data. The contraction-responses of GA with pre-treatment of ponatinib (200 nmol/L) for 20 min to PE-induced constriction by (H) the typical recording and (I) statistical data. N = 6. Data are expressed as mean ± SEM.
Figure 3. SUMO inhibitors increase the expression of NO by activating the ERK5/KLF2/eNOS pathway in HUVECs
(A)The level of NO (green) was detected by immunofluorescence after treatment with SUMO inhibitors with or without ponatinib. Relative fluorescence unit of eNOS in HUVECs after incubation with (B) Tannic acid (TA), (C) 2-D08, and (D) Ginkgolic acid (GA) with or without ponatinib. N = 5. Data are expressed as mean ± SEM. ***P < 0.001, compared to the control group; ##P < 0.01, ###P < 0.001, compared to the ponatinib group; ^P < 0.05, ^^P < 0.01 compared to the TA, 2-D08 or GA group. (E) The colocalization of ERK5 (green) and SUMO1 (red) was observed under a confocal microscope. Scale bar: 20 μm. (F-H) The expression levels of KLF2 (F-G) and p-eNOS (H) measured by Western blot analysis and statistical data. (I) The transcriptional activity of eNOS promotor assayed by ChIP-PCR. (J) The relative mRNA levels of eNOS detected by real-time PCR; N = 3. *P < 0.05, **P < 0.01, ***P < 0.001, compared to the control group; #P < 0.05, ##P < 0.01, compared to the ponatinib group; ^^P < 0.01 compared to the TA group.
Figure 4. Tannic acid decreases blood pressure and improves vascular dysfunction in WKY rats and spontaneous hypertension rat (SHR)
(A-D) Blood pressure was monitored in SHR after administration with (100 mg/kg) and Nifedipine (0.2 mg/kg) for 4 weeks, Nifedipine was used as a positive control drug. (E) The contents of NO and (F) ET-1 in serum of SHR after Tannic acid (TA) administration for 4 weeks. (G-I) Representative traces showed the effect of TA on acetylcholine (0.01 to 10 µmol/L)-induced dilation and phenylephrine (PE) (0.5 to 10 µmol/L)-induced contraction in isolated mesenteric artery rings. (J) Contraction-response curves of PE-induced in mesenteric artery rings in response to TA. (K) Dilation-response curves to ACh-induced in mesenteric artery rings in response to TA; N = 7. (L-O) The expression level of Krüppel-like factor 2 (KLF2) and p-eNOS in mesenteric artery were measured by Western blot analysis N = 3. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, compared to WKY group; #P < 0.05, ##P < 0.01, ###P < 0.001, compared to SHR group.
-
[1] Mills K T, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol, 2020; 16(4): 223-237. doi: 10.1038/s41581-019-0244-2 [2] Poulter N R, Prabhakaran D, Caulfield M. Hypertension. Lancet, 2015; 386(9995): 801-812. doi: 10.1016/S0140-6736(14)61468-9 [3] Dehnavi S, Sadeghi M, Penson P E, et al. The role of protein SUMOylation in the pathogenesis of atherosclerosis. J Clin Med, 2019; 8(11): 1856. doi: 10.3390/jcm8111856 [4] Kukkula A, Ojala V K, Mendez L M, et al. Therapeutic potential of targeting the SUMO pathway in cancer. Cancers (Basel), 2021; 13(17): 4402. doi: 10.3390/cancers13174402 [5] Paudel R, Fusi L, Schmidt M. The MEK5/ERK5 pathway in health and disease. Int J Mol Sci, 2021; 22(14): 7594. doi: 10.3390/ijms22147594 [6] Roberts O L, Holmes K, Muller J, et al. ERK5 and the regulation of endothelial cell function. Biochem Soc Trans, 2009; 37(Pt 6): 1254-1259. [7] Woo C H, Shishido T, Mcclain C, et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res, 2008; 102(5): 538-545. doi: 10.1161/CIRCRESAHA.107.156877 [8] Shishido T, Woo C H, Ding B, et al. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res, 2008; 102(11): 1416-1425. doi: 10.1161/CIRCRESAHA.107.168138 [9] Erazo T, Espinosa-gil S, Dieguez-martinez N, et al. SUMOylation is required for ERK5 nuclear translocation and ERK5-mediated cancer cell proliferation. Int J Mol Sci, 2020; 21(6): 2203. doi: 10.3390/ijms21062203 [10] Paez-mayorga J, Chen A L, Kotla S, et al. Ponatinib activates an inflammatory response in endothelial cells via ERK5 SUMOylation. Front Cardiovasc Med, 2018; 5: 125. doi: 10.3389/fcvm.2018.00125 [11] Yang T, Shu F, Yang H, et al. YY1: A novel therapeutic target for diabetic nephropathy orchestrated renal fibrosis. Metabolism, 2019; 96: 33-45. doi: 10.1016/j.metabol.2019.04.013 [12] Wu W, Geng P, Zhu J, et al. KLF2 regulates eNOS uncoupling via Nrf2/HO-1 in endothelial cells under hypoxia and reoxygenation. Chem Biol Interact, 2019; 305: 105-111. doi: 10.1016/j.cbi.2019.03.010 [13] Huai R, Han X, Wang B, et al. Vasorelaxing and antihypertensive effects of 7, 8-dihydroxyflavone. Am J Hypertens, 2014; 27(5): 750-760. doi: 10.1093/ajh/hpt220 [14] Vertegaal A C O. Signalling mechanisms and cellular functions of SUMO. Nat Rev Mol Cell Biol, 2022; 23(11): 715-731. doi: 10.1038/s41580-022-00500-y [15] Brown M D, Sacks D B. Protein scaffolds in MAP kinase signalling. Cell Signal, 2009; 21(4): 462-469. doi: 10.1016/j.cellsig.2008.11.013 [16] Ohashi Y, Kawashima S, Hirata K, et al. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest, 1998; 102(12): 2061-2071. doi: 10.1172/JCI4394 [17] Komaravolu R K, Adam C, Moonen J R, et al. Erk5 inhibits endothelial migration via KLF2-dependent down-regulation of PAK1. Cardiovasc Res, 2015; 105(1): 86-95. doi: 10.1093/cvr/cvu236 [18] Fulton D J. Transcriptional and posttranslational regulation of eNOS in the endothelium. Adv Pharmacol, 2016; 77: 29-64. [19] Pazoki R, Dehghan A, Evangelou E, et al. Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation, 2018; 137(7): 653-661. doi: 10.1161/CIRCULATIONAHA.117.030898 [20] Weinberger M H. The cold pressor test: a new predictor of future hypertension? Arch Intern Med, 2008; 168(16): 1732. doi: 10.1001/archinte.168.16.1732 [21] Zhao Q, Gu D, Lu F, et al. Blood pressure reactivity to the cold pressor test predicts hypertension among Chinese adults: the gensalt study. Am J Hypertens, 2015; 28(11): 1347-1354. doi: 10.1093/ajh/hpv035 [22] Radin J M, Neems D, GogliA R, et al. Inverse correlation between daily outdoor temperature and blood pressure in six US cities. Blood Press Monit, 2018; 23(3): 148-152. doi: 10.1097/MBP.0000000000000322 [23] Kim J Y, Jung K Y, Hong Y S, et al. The relationship between cold exposure and hypertension. J Occup Health, 2003; 45(5): 300-306. doi: 10.1539/joh.45.300 [24] Lewington S, Li L, Sherliker P, et al. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. J Hypertens, 2012; 30(7): 1383-1391. doi: 10.1097/HJH.0b013e32835465b5 [25] Fregly M J, Kikta D C, Threatte R M, et al. Development of hypertension in rats during chronic exposure to cold. J Appl Physiol (1985), 1989; 66(2): 741-749. doi: 10.1152/jappl.1989.66.2.741 [26] Kanayama N, Tsujimura R, She L, et al. Cold-induced stress stimulates the sympathetic nervous system, causing hypertension and proteinuria in rats. J Hypertens, 1997; 15(4): 383-389. doi: 10.1097/00004872-199715040-00009 [27] Pan Z, Zhuang J, Zhu C, et al. Impacts of cold-stress stimulation on mice pregnancy. Am J Hypertens, 2023; 36(6): 348-353. doi: 10.1093/ajh/hpad003 [28] Zhu Z, Zhu S, Zhu J, et al. Endothelial dysfunction in cold-induced hypertensive rats. Am J Hypertens, 2002; 15(2 Pt 1): 176-180. [29] Chen P G, Sun Z. AAV Delivery of endothelin-1 shRNA attenuates cold-induced hypertension. Hum Gene Ther. 2017; 28(2): 190-199. doi: 10.1089/hum.2016.047 [30] Brackett C M, Blagg B S J. Current status of SUMOylation inhibitors. Curr Med Chem, 2021; 28(20): 3892-3912. doi: 10.2174/0929867327666200810135039 [31] Langston S P, Grossman S, England D, et al. Discovery of TAK-981, a first-in-class inhibitor of SUMO-activating enzyme for the treatment of cancer. J Med Chem, 2021; 64(5): 2501-2520. doi: 10.1021/acs.jmedchem.0c01491 [32] Chen Z, Cui Q, Cooper L, et al. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci, 2021; 11(1): 45. doi: 10.1186/s13578-021-00564-x [33] Qiu F, Dong C, Liu Y, et al. Pharmacological inhibition of SUMO-1 with ginkgolic acid alleviates cardiac fibrosis induced by myocardial infarction in mice. Toxicol Appl Pharmacol, 2018; 15(345): 1-9. [34] Esparis-ogando A, Diaz-rodriguez E, Montero J C, et al. Erk5 participates in neuregulin signal transduction and is constitutively active in breast cancer cells overexpressing ErbB2. Mol Cell Biol, 2002; 22(1): 270-285. doi: 10.1128/MCB.22.1.270-285.2002 [35] Buschbeck M, Ullrich A. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J Biol Chem, 2005; 280(4): 2659-2667. doi: 10.1074/jbc.M412599200 [36] Nigro P, Abe J, Woo C H, et al. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood, 2010; 116(11): 1971-1979. doi: 10.1182/blood-2010-02-269134 [37] Angolano C, Kaczmarek E, Essayagh S, et al. A20/TNFAIP3 increases ENOS expression in an ERK5/KLF2-dependent manner to support endothelial cell health in the face of inflammation. Front Cardiovasc Med, 2021; 8: 651230. doi: 10.3389/fcvm.2021.651230 [38] Li Q, Chen Y, Zhang X, et al. Scutellarin attenuates vasospasm through the Erk5-KLF2-eNOS pathway after subarachnoid hemorrhage in rat. J Clin Neurosci, 2016; 34(2): 64-70. [39] Lee G H, Park J S, Jin S W, et al. Betulinic acid induces eNOS expression via the AMPK-dependent KLF2 signaling pathway. J Agric Food Chem, 2020; 68(49): 14523-14530. doi: 10.1021/acs.jafc.0c06250 [40] Heo K S, Fujiwara K, Abe J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ J, 2011; 75(12): 2722-2730. doi: 10.1253/circj.CJ-11-1124 [41] Suzawa M, Miranda D A, Ramos K A, et al. A gene-expression screen identifies a non-toxic sumoylation inhibitor that mimics SUMO-less human LRH-1 in liver. Elife, 2015; 44: e09003. -


 投稿系统
投稿系统


 下载:
下载: