Vitamin D deficiency and increased inflammatory factor intercellular cell adhesion molecule-1 indicate severe leukoaraiosis in northern China
doi: 10.1515/fzm-2024-0011
-
Abstract:
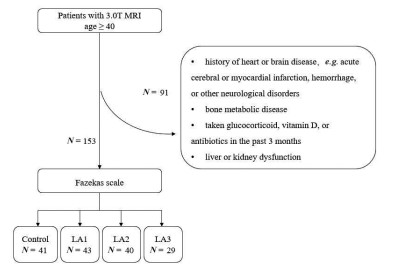
Background and objective Commonly plaguing in the frigid zone of the world, vitamin D deficiency, as indicated by low levels of 25-hydroxyvitamin D, exacerbated inflammatory responses and impaired endothelial function. Leukoaraiosis (LA) is a prevalent cause of cognitive dysfunction in the elderly and is potentially associated with inflammatory responses. This study aimed to investigate the impact of vitamin D on the severity of LA. Methods Patients with LA were categorized based on 3.0 T brain MRI findings into mild (N = 43), moderate (N = 40), or severe groups (N = 29) using the Fazekas scale (scoring 1-6). A control group consisting of 41 healthy individuals was included. Serum fibrinogen C, homocysteine, plasma 25-hydroxyvitamin D, and intercellular cell adhesion molecule-1 (ICAM-1) levels were measured using ELISA. Results All LA severity groups exhibited lower plasma 25-hydroxyvitamin D levels compared to the control group, with a more pronounced decrease observed as LA severity increased. Low plasma 25-hydroxyvitamin D was identified as an independent risk factor for LA (P < 0.05) according to Multiple logistic regression analysis. Additionally, a negative association was observed between 25-hydroxyvitamin D and vascular inflammatory factor ICAM-1. Conclusions Disease severity positively correlated with levels of the inflammatory marker ICAM-1, worsening as plasma 25-hydroxyvitamin D concentration decreased. Low 25-hydroxyvitamin D emerged as an independent risk factor for LA, potentially exacerbating the inflammatory response. These findings suggest 25-hydroxyvitamin D supplementation as a potential therapeutic approach for LA. -
Table 1. Statistical methods for different data
Data Statistical methods Normal distribution data Analysis of variance (ANOVA) Non normal distribution data Kruskal-Wallis H test Enumeration data Chi-squared test (χ2) Fisher's exact test Multivariate analysis Multiple logistic regression analysis Correlation analysis Spearman's rho analysis Table 2. Basic medical history of the Leukoaraiosis (LA) and control groupsa
Control (N = 41) LA-mild (N = 43) LA-moderate (N =40) LA-severe (N =29) χ2/H P Gender M/F, N/N 18/23 22/21 14/26 14/15 2.408 0.492 Age, y 63.00 (59.50, 70.00) 65.00 (60.00, 68.00) 66.00 (62.00, 70.75) 71.00 (63.00, 79.00)a 10.50 0.015 LI 28 (68) 36 (83) 39 (98) 29 (100)a 20.302 < 0.001 Smoking 6 (15) 15 (35) 78 (20) 6 (21) 5.269 0.153 Drinking 1 (2) 12 (28)a 2 (5)b 3 (10) 16.048 0.001 Diabetes 4 (10) 8 (19) 10 (25) 10 (34) 6.856 0.077 Hypertension 15 (37) 20 (47) 23 (58) 20 (69)a 8.177 0.042 CHD 6 (15) 10 (23) 8 (20) 4 (14) 1.546 0.672 Data were presented as N (%), unless indicated otherwise. Age was not normally distributed among the 4 groups and was summarized using the median (interquartile 25, interquartile 75) and subjected to the Kruskal-Wallis H test.aCompared with control group, P < 0.05. bCompared with LA-mild, P < 0.05. CHD, coronary heart disease; LI, lacunar infarction. Table 3. Blood indicators of the Leukoaraiosis (LA) and control groups
Control (N = 41) LA-mild (N = 43) LA-moderate (N =40) LA-severe (N =29) F P Apo B, g/L 1.03 ± 0.29 0.92 ± 0.23 0.96 ± 0.25 1.02 ± 0.31 1.503 0.216 LDL, mmol/L 3.00 ± 0.85 2.65 ± 0.70 2.73 ± 0.84 2.95 ± 0.96 1.631 0.185 ALB, g/L 43.35 ± 3.83 43.72 ± 3.38 43.69 ± 3.97 41.73 ± 3.88 1.992 0.118 25(OH)D, ng/mL 33.59 ± 19.21 25.03 ± 13.63a 21.69 ± 9.86a 17.98 ± 8.70a, b 8.409 < 0.001 Data were presented as mean±SD. aP < 0.05 compared with control group; bP < 0.05 compared with LA-mild group. Apo B, apolipoprotein B; ALB, albumin; LDL, low-density lipoprotein; 25(OH)D, 25-hydroxyvitamin D. Table 4. Blood indicators of the Leukoaraiosis (LA) and control groups
Control (N = 41) LA-mild (N = 43) LA-moderate (N =40) LA-severe (N =29) H P HcyH, µmol/L 10.48 (8.56, 12.86) 11.59 (9.52, 13.25) 10.50 (9.02, 13.11) 10.17 (8.38, 13.60) 2.480 0.479 ApoA1, g/L 1.25 (1.11, 1.37) 1.25 (1.11, 1.36) 1.28 (1.08, 1.45) 1.27 (1.10, 1.45) 0.040 0.998 TC, mmol/L 4.78 (4.21, 5.71) 4.45 (3.86, 5.16) 4.73 (3.74, 5.63) 5.11 (3.92, 5.79) 4.292 0.232 TG, mmol/L 1.35 (1.02, 1.74) 1.12 (0.86, 1.90) 1.46 (1.08, 2.24) 1.46 (1.08, 2.24) 4.820 0.185 HDL, mmol/L 1.28 (1.05, 1.63) 1.29 (1.08, 1.51) 1.34 (1.07, 1.57) 1.24 (1.08, 1.53) 0.303 0.960 FBG, mmol/L 5.37 (4.98, 5.77) 5.48 (4.89, 6.09) 5.35 (4.94, 6.67) 5.52 (4.88, 7.37) 1.701 0.637 Cr, µmol/L 72.00 (64.50, 92.00) 71.00 (65.00, 80.00) 71.50 (61.25, 85.75) 72.00 (62.00, 89.00) 0.311 0.958 UA, µmol/L 325.00 (277.80, 386.95) 307.00 (261.40, 404.90) 300.05 (236.00, 364.01) 308.80 (250.05, 385.95) 1.546 0.672 FBG-C, g/L 2.93 (2.66, 3.36) 2.86 (2.65, 3.58) 3.18 (2.98, 3.63) 3.56 (3.14, 3.91)a, b 17.196 0.001 ICAM-1, ng/mL 439.86 (346.10, 628.29) 584.38 (432.30, 632.81) 581.26 (514.33, 669.53) 620.31 (596.04, 757.30)a, b 21.247 < 0.001 Data were presented as median (interquartile 25, interquartile 75). aP < 0.05 compared with control group; bP < 0.05 compared with LA-mild group. Abbreviations: ApoA1, apolipoprotein A; Cr, creatinine; FBG, fasting blood glucose; HcyH, homocysteine; HDL, high-density lipoprotein; TC, total cholesterol; TG, triglycerides; UA, uric acid. Table 5. Variable assignment table for multiple logistic regression
Variable Name Variable assignment Dependent variable Leukoaraiosis severity Severe = 1, Moderate =2, Mild = 3, Not ill = 4 Independent variable Gender Male = 1, Female = 0 Lacunar infarction Yes = 1, No = 0 Alcohol consumption Yes = 1, No = 0 Hypertension Yes = 1, No = 0 Age, y Continuous 25-hydroxyvitamin D Continuous ICAM-1 Continuous FBG-C fibrinogen Continuous Table 6. Multiple logistic regression analysis of Leukoaraiosis
b SE Wald χ2 P OR (95% CI) Gender 0.696 0.478 2.120 0.145 2.007 (0.786, 5.124) Lacunar infarction 1.432 0.675 4.499 0.034 4.187 (1.115 15.728) Alcohol consumption 3.266 1.234 7.008 0.008 26.219 (2.335, 294.387) Hypertension 0.308 0.476 0.419 0.517 1.361 (0.535, 3.459) Age, y 0.017 0.032 0.304 0.581 1.017 (0.924, 1.045) 25-hydroxyvitamin D –0.055 0.016 12.345 < 0.001 0.946 (1.025, 1.090) ICAM-1 0.005 0.001 9.616 0.002 1.005 (0.992, 0.998) FBG-C fibrinogen 0.031 0.350 0.008 0.929 1.031 (0.488, 1.925) b, regression coefficient; CI, confidence interval; OR, odds ratio; SE, standard error. -
[1] Wardlaw J M, Smith E E, Biessels G J, et al. Neuroimaging standards neurodegeneration. Lancet Neurol, 2013; 12(8): 822-838. doi: 10.1016/S1474-4422(13)70124-8 [2] Cannistraro R J, Badi M, Eidelman B H, et al. CNS small vessel disease: A clinical review. Neurology, 2019; 92(24): 1146-1156. doi: 10.1212/WNL.0000000000007654 [3] Su N, Zhai F F, Zhou LX, et al. Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community-dwelling populations. Front Aging Neurosci, 2017; 9: 313. doi: 10.3389/fnagi.2017.00313 [4] Zhi N, Zhang L, Wang Y, et al. Modified cerebral small vessel disease score is associated with vascular cognitive impairment after lacunar stroke. Aging (Albany NY), 2021; 13(7): 9510-9521. doi: 10.18632/aging.202438 [5] van der Holst H M, van Uden I W, Tuladhar A M, et al. Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the radboud university nijmegen diffusion tensor and magnetic resonance cohort (RUN DMC) study. JAMA Neurol, 2016; 73(4): 402-409. doi: 10.1001/jamaneurol.2015.4560 [6] Wardlaw J M, Chappell F M, Valdes Hernandez M D C, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology, 2017; 89(10): 1003-1010. doi: 10.1212/WNL.0000000000004328 [7] Jiaerken Y, Luo X, Yu X, et al. Microstructural and metabolic changes in the longitudinal progression of white matter hyperintensities. J Cereb Blood Flow Metab, 2019; 39(8): 1613-1622. doi: 10.1177/0271678X18761438 [8] Howard D P J, Gaziano L, Rothwell P M, et al. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol, 2021; 20(3): 193-202. doi: 10.1016/S1474-4422(20)30484-1 [9] Bernbaum M, Menon B K, Fick G, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab, 2015; 35(10): 1610-1615. doi: 10.1038/jcbfm.2015.92 [10] Promjunyakul N O, Dodge H H, Lahna D, et al. Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology, 2018; 90(24): e2119-e2126. doi: 10.1212/WNL.0000000000005684 [11] Philips T Rothstein J D. Oligodendroglia: metabolic supporters of neurons. J Clin Invest, 2017; 127(9): 3271-3280. doi: 10.1172/JCI90610 [12] Sargurupremraj M, Suzuki H, Jian X, et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nat Commun, 2020; 11(1): 6285. doi: 10.1038/s41467-020-19111-2 [13] Gardener H, Caunca M, Dong C, et al. Ideal cardiovascular health and biomarkers of subclinical brain aging: the northern Manhattan study. J Am Heart Assoc, 2018; 7(16): e009544. doi: 10.1161/JAHA.118.009544 [14] Shu Z, Xu Y, Shao Y, et al. Radiomics from magnetic resonance imaging may be used to predict the progression of white matter hyperintensities and identify associated risk factors. Eur Radiol, 2020; 30(6): 3046-3058. doi: 10.1007/s00330-020-06676-1 [15] Shu Z Y, Shao Y, Xu Y Y, et al. Radiomics nomogram based on MRI for predicting white matter hyperintensity progression in elderly adults. J Magn Reson Imaging, 2020; 51(2): 535-546. doi: 10.1002/jmri.26813 [16] Whitman G T, Tang Y, Lin A, et al. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology, 2001; 57(6): 990-994. doi: 10.1212/WNL.57.6.990 [17] Lin L, Zhang Y, Zeng Q, et al. Atherosclerosis, inflammatory factor changes, cognitive disorder and vascular endothelial functions in patients with different grades of leukoaraiosis. Clin Hemorheol Microcirc, 2019; 73(4): 591-597. doi: 10.3233/CH-190597 [18] Xue J, Wu Z, Gong S, et al. High-dose atorvastatin improves vascular endothelial function in patients with leukoaraiosis. J Clin Lab Anal, 2020; 34(3): e23081. doi: 10.1002/jcla.23081 [19] Arba F, Giannini A, Piccardi B, et al. Small vessel disease and biomarkers of endothelial dysfunction after ischaemic stroke. Eur Stroke J, 2019; 4(2): 119-126. doi: 10.1177/2396987318805905 [20] Cao G Z, Hou J Y, Zhou R, et al. Single-cell RNA sequencing reveals that VIM and IFITM3 are vital targets of Dengzhan Shengmai capsule to protect against cerebral ischemic injury. J Ethnopharmacol, 2023; 311: 116439. doi: 10.1016/j.jep.2023.116439 [21] Johnson C R, Thacher T D. Vitamin D: immune function, inflammation, for research into small vessel disease and its contribution to ageing and infections and auto-immunity. Paediatr Int Child Health, 2023; 43(4): 29-39. doi: 10.1080/20469047.2023.2171759 [22] Zhou A, Hypponen E. Vitamin D deficiency and C-reactive protein: a bidirectional Mendelian randomization study. Int J Epidemiol, 2023; 52(1): 260-271. doi: 10.1093/ije/dyac087 [23] Jin D, Zhu D M, Hu H L, et al. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr Metab (Lond), 2020; 17: 57. doi: 10.1186/s12986-020-00455-x [24] Meng J, Xu Y R, Miao X H, et al. A retrospective study on serum 25 (OH) D level of the sampled population in a hospital in Heilongjiang province. Chinese Journal of Osteoporosis, 2022; 28(1): 62-69. [25] Li L, Zhao YX, Sun G W, et al. Analysis of the content of adult 25-hydroxyvitamin D in Heilongjiang Province. Cardiovascular Disease Journal of Integrated Traditional Chinese and Western Medicine (Electronic), 2018; 6(27): 187, 190. [26] Fazekas F, Deisenhammer F, Strasser-Fuchs S, et al. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Austrian Immunoglobulin in Multiple Sclerosis Study Group. Lancet, 1997; 349(9052): 589-593. doi: 10.1016/S0140-6736(96)09377-4 [27] Guan J, Yan C, Gao Q, et al. Analysis of risk factors in patients with leukoaraiosis. Medicine (Baltimore), 2017; 96(8): e6153. doi: 10.1097/MD.0000000000006153 [28] Leung L Y, Bartz T M, Rice K, et al. Blood pressure and heart rate measures associated with increased risk of covert brain infarction and worsening leukoaraiosis in older adults. Arterioscler Thromb Vasc Biol, 2017; 37(8): 1579-1586. doi: 10.1161/ATVBAHA.117.309298 [29] Levit A, Hachinski V, Whitehead S N. Neurovascular unit dysregulation, white matter disease, and executive dysfunction: the shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience, 2020; 42(2): 445-465. doi: 10.1007/s11357-020-00164-6 [30] Drieu A, Lanquetin A, Levard D, et al. Alcohol exposure-induced neurovascular inflammatory priming impacts ischemic stroke and is linked with brain perivascular macrophages. JCI Insight, 2020; 5(4): e129226. doi: 10.1172/jci.insight.129226 [31] Bustamante A, Lopez-Cancio E, Pich S, et al. Blood biomarkers for the early diagnosis of stroke: the stroke-chip study. Stroke, 2017; 48(9): 2419-2425. doi: 10.1161/STROKEAHA.117.017076 [32] Bembenek J P, Niewada M, Siudut J, et al. Fibrin clot characteristics in acute ischaemic stroke patients treated with thrombolysis: the impact on clinical outcome. Thromb Haemost, 2017; 117(7): 1440-1447. doi: 10.1160/TH16-12-0954 [33] Ashouri R, Fangman M, Brielmaier J, et al. Nutritional supplementation of naturally occurring vitamin D to improve hemorrhagic stroke outcomes. Front Neurol, 2021; 12: 670245. doi: 10.3389/fneur.2021.670245 [34] Fu Y, Yan Y. Emerging role of immunity in cerebral small vessel disease. Front Immunol, 2018; 9: 67. doi: 10.3389/fimmu.2018.00067 [35] Shi L, Zhang Q, Song S N, et al. Correlation between 25-hydroxyvitamin D level and arterial elasticity in middle-aged and elderly cadres in Guiyang, China: A retrospective observational study. Medicine (Baltimore), 2021; 100(18): e25826. doi: 10.1097/MD.0000000000025826 [36] Miao J, Bachmann K N, Huang S, et al. Effects of vitamin D supplementation on cardiovascular and glycemic biomarkers. J Am Heart Assoc, 2021; 10(10): e017727. doi: 10.1161/JAHA.120.017727 [37] An J H, Cho D H, Lee G Y, et al. Effects of vitamin D supplementation on CD4(+) T cell subsets and mtor signaling pathway in high-fat-diet-induced obese mice. Nutrients, 2021; 13(3): 796. doi: 10.3390/nu13030796 [38] Boontanrart M, Hall S D, Spanier J A, et al. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol, 2016; 292: 126-136. doi: 10.1016/j.jneuroim.2016.01.015 [39] Evans M A, Kim H A, Ling Y H, et al. Vitamin D3 supplementation reduces subsequent brain injury and inflammation associated with ischemic stroke. Neuromolecular Med, 2018; 20(1): 147-159. doi: 10.1007/s12017-018-8484-z -


 投稿系统
投稿系统


 下载:
下载: