Notum protects against myocardial infarction-induced heart dysfunction by alleviating cardiac fibrosis
doi: 10.2478/fzm-2024-0005
-
Abstract:
Background and Objective Cardiac fibrosis is a pathological reparative process that follows myocardial infarctionand is associated with compromised cardiac systolic and reduced cardiac compliance. The Wnt signaling pathway is closely implicated in organ fibrosis, and Notum, a highly conserved secreted inhibitor, modulates Wnt signaling. The objective of this study was to explore the role and mechanism of Notum in cardiac fibrosis. Methods A mouse model of cardiac remodeling was established through left coronary artery ligation surgery, with the addition of Notum injection following myocardial infarction surgery. The protective effect of Notum on myocardial infarction was assessed by evaluating cardiac function, including survival rate, echocardiographic assessment, and cardiac contraction analyses. Inflammatory cell necrosis and infiltration were confirmed through H & E and Masson staining. The expression of fibrosis-related genes and β-catenin pathway markers was detected using Western blot quantificational RT-PCR (qRT-PCR). Additionally, EdU, wound healing, and immunofluorescence staining analyses were performed to detect the effect of Notum's in transforming growth factor beta-1 (TGF-β1) induced myofibroblast transformation. Results The administration of Notum treatment resulted in enhanced survival rates, improved cardiac function, and decreased necrosis and infiltration of inflammatory cells in mice subjected to left coronary artery ligation. Furthermore, Notum effectively impeded the senescence of cardiac fibroblasts and hindered their pathological transformation into cardiac fibroblasts. Additionally, it significantly reduced collagen production and attenuated the activation of the Wnt/β-catenin pathway. Our preliminary investigations successfully demonstrated the therapeutic potential of Notum in both fibroblasts in vitro and in a mouse model of myocardial infarction-induced cardiac fibrosis in vivo. Conclusion Notum inhibition of the Wnt/β-catenin signaling pathway and cardiac fibroblast senescence ultimately hampers the onset of cardiac fibrosis. Our findings suggest that Notum could represent a new therapeutic strategy for the treatment of cardiac fibrosis. -
Key words:
- cardiac fibrosis /
- Notum /
- Wnt/β-catenin /
- senescence /
- myocardial infarction
-
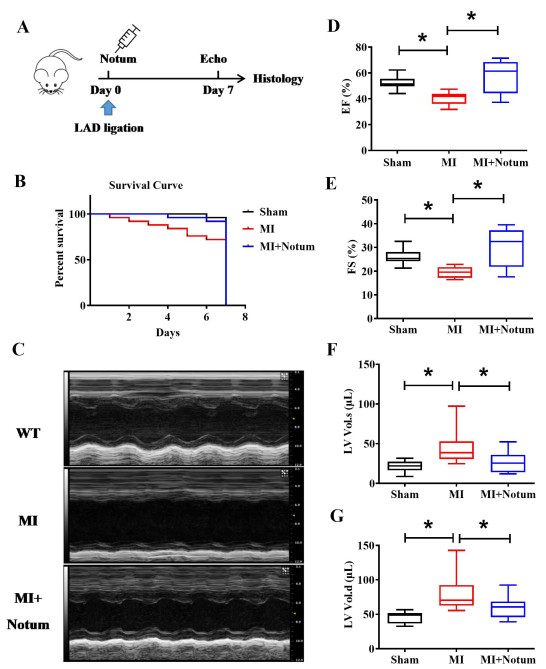
Figure 1. Notum increases survival and improves cardiac function in myocardial injury (MI) mice. (A) Notum treatment protocol. (B) Survival curves of specified groups of mice. (C) Echocardiography was applied to assessing the heart function of MI mice after treatment with Notum (N = 5), as indicated by ejection fraction (EF). (D) fraction shortening (FS). (E) left ventricular systolic volume (LV.Vol.s) (F), and left ventricular diastolic volume (Lv.vol.d) (G). Data are presented as mean ± SEM. *P < 0.01. Survival distributions were estimated by the Kaplan-Meier method and compared by log-rank test.
Figure 2. Notum attenuates myocardial injury (MI)-induced cardiac fibrosis. (A-B) Representative images of H & E and Masson's trichrome-stained sections. The left panel shows the whole heart section. Scale bars: 500 µm. The right panel represents enlarged view of selected fields. Scale bars: 50 µm. N = 5-7. (C) Western blot analysis of fibrosis-related proteins (FN1 and α-SMA) in the hearts. N = 5-6. (D-E) Immunohistochemistry staining of FN1 and α-SMA in sham, MI and MI+Notum mice. The left panel is an image for the whole heart section. Scale bars: 500 µm. The right panel represents enlarged view of selected fields. Scale bars: 50 µm. N = 4-7. (F) Relative levels of FN1, Col1α1, Col3α1 mRNAs analyzed by qRT-PCR. N = 5-6, *P < 0.01 vs. Sham or MI. Data are presented as mean ± SEM.
Figure 3. Notum abrogates cardiac fibroblasts activation induced by TGF-β1. (A) Western blot analysis of FN1 and Collagen 1 in cardiac fibroblasts after 24 h treatment with TGF-β1 and Notum. N = 6. (B) qRT-PCR analysis of the expression of FN1, Col 1α1, Col 3α1 and α-SMA mRNAs in cardiac fibroblasts. N = 5–6. (C) EDU staining demonstrates the effect of Notum on TGF-β1-induced cardiac fibroblasts proliferation. Scale bars: 50 µm; N = 8. (D) Wound healing scratch assay evaluates the effect of Notum on TGF-β1-induced migration in cardiac fibroblasts. Scale bars: 200 µm; N = 8. (E) Effect of Notum on cardiac fibroblast-myofibroblast transition, as measured by immunofluorescence. Scale bars: 50 µm. Data are presented as mean ± SEM. *P < 0.01 vs. CTL; #P < 0.01 vs. TGF-β1.
Figure 4. Notum inhibits Wnt/β-catenin signaling activation in cardiac fibroblasts. (A) Immunohistochemistry showing decreased protein expression of β-catenin in myocardial injury (MI)+Notum-treated mice compared to MI mice. Scale bar: 50 µm; N = 3. (B) Western blot analysis of β-catenin in MI model after injection with Notum. N = 4-6, *P < 0.01. (C) qRT-PCR analysis of the mRNA level of β-catenin in mice. N = 6, *P < 0.01 vs. Sham or MI. (D) The protein levels of β-catenin and GSK-3β measured by Western blot in TGF-β1-induced cardiac fibrogenesis after Notum treatment. N = 3-5, *P < 0.01 vs. Control or TGF-β1 (E) qRT-PCR analysis of the expression of β-catenin mRNA in vitro. N = 3, *P < 0.01. Data are presented as mean ± SEM.
Figure 5. The effect of Notum on cardiac fibroblasts senescence. (A) Western blot analysis of the protein level of p53 in cardiac fibroblasts treated with H2O2 for 2h after 22h treatment of Notum. N = 5, *P < 0.01. (B) and (C) Relative mRNA levels of p21 and p16 analyzed by qRT-PCR. N = 3-5, *P < 0.01. (D) Immunohistochemical staining showing the expression changes of p16 in mice. Scale bars: 50 µm; N = 4-5. Data are presented as mean ± SEM.
Figure 6. Schematic diagram for the proposed mechanism for the anti-fibrotic effect of Notum in the heart. Upon receiving stimuli, cardiac fibroblasts undergo activation of the canonical WNT/β-catenin signaling pathway, promoting the fibrogenesis and senescence of cardiac fibroblasts, ultimately leading to cardiac fibrosis (CF). This triggers Notum, a carboxylesterase, to exert an anti-fibrotic effect via inhibiting WNT/β-catenin signaling.
-
[1] Heymans S, Gonzalez A, Pizard A, et al. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail, 2015; 17(8): 764-771. doi: 10.1002/ejhf.312 [2] Claeys M J, Rajagopalan S, Nawrot T S, et al. Climate and environmental triggers of acute myocardial infarction. Eur Heart J, 2017; 38(13): 955-960. [3] Creemers E E, Pinto Y M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res, 2011; 89(2): 265-272. doi: 10.1093/cvr/cvq308 [4] de Boer R A, De Keulenaer G, Bauersachs J, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail, 2019; 21(3): 272-285. doi: 10.1002/ejhf.1406 [5] Shih Y C, Chen C L, Zhang Y, et al. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ Res, 2018; 122(2): 1052-1068. doi: 10.1161/CIRCRESAHA.117.312130 [6] Driesen R B, Nagaraju C K, Abi-Char J, et al. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res, 2014; 101(3): 411-22. doi: 10.1093/cvr/cvt338 [7] Yu J, Seldin M M, Fu K, et al. Topological arrangement of cardiac fibroblasts regulates cellular plasticity. Circ Res, 2018; 123(1): 73-85. doi: 10.1161/CIRCRESAHA.118.312589 [8] Travers JG, Kamal FA, Robbins J, et al. Cardiac fibrosis: the fibroblast awakens. Circ Res, 2016; 118(6): 1021-1040. doi: 10.1161/CIRCRESAHA.115.306565 [9] Kong P, Christia P, Frangogiannis N G. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci, 2014; 71(4): 549-574. doi: 10.1007/s00018-013-1349-6 [10] Bernaba B N, Chan J B, Lai C K et al. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol, 2010; 19(5): 308-311. doi: 10.1016/j.carpath.2009.07.004 [11] Wu H, Tang L X, Wang X M, et al. Porcupine inhibitor CGX1321 alleviates heart failure with preserved ejection fraction in mice by blocking WNT signaling. Acta Pharmacol Sin, 2023; 44(6): 1149-1160. doi: 10.1038/s41401-022-01025-y [12] Qian L, Hong J, Zhang Y, et al. Downregulation of S100A4 Alleviates Cardiac Fibrosis via Wnt/β-Catenin Pathway in Mice. Cell Physiol Biochem, 2018; 46(6): 2551-2560. doi: 10.1159/000489683 [13] Weber K T, Sun Y, Bhattacharya S K, et al. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol, 2013; 10(1): 15-26. doi: 10.1038/nrcardio.2012.158 [14] Frangogiannis N G. Regulation of the inflammatory response in cardiac repair. Circ Res, 2012; 110(1): 159-173. doi: 10.1161/CIRCRESAHA.111.243162 [15] Pentinmikko N, Iqbal S, Mana M, et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature, 2019; 571(7765): 398-402. doi: 10.1038/s41586-019-1383-0 [16] Blyszczuk P, Berthonneche C, Behnke S, et al. Nitric oxide synthase 2 is required for conversion of pro-fibrogenic inflammatory CD133(+) progenitors into F4/80(+) macrophages in experimental autoimmune myocarditis. Cardiovasc Res, 2013; 97(2): 219-229. doi: 10.1093/cvr/cvs317 [17] Porter K E, Turner N A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther, 2009; 123(2): 255-278. doi: 10.1016/j.pharmthera.2009.05.002 [18] Yang D, Fu W, Li L, et al. Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction. Clin Sci (Lond), 2017; 131(24): 2919-2932. doi: 10.1042/CS20171256 [19] Tao H, Yang J J, Shi K H, et al. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism, 2016; 65(2): 30-40. doi: 10.1016/j.metabol.2015.10.013 [20] Dzialo E, Rudnik M, Koning R I, et al. WNT3a and WNT5a transported by exosomes activate WNT signaling pathways in human cardiac fibroblasts. Int J Mol Sci, 2019; 20(6): 1436. doi: 10.3390/ijms20061436 [21] Duan J, Gherghe C, Liu D, et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J, 2012; 31(2): 429-442. doi: 10.1038/emboj.2011.418 [22] Xiang F L, Fang M, Yutzey K E. Loss of beta-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun, 2017; 8(1): 712. doi: 10.1038/s41467-017-00840-w [23] Miao J, Liu J, Niu J, et al. Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell, 2019; 18(5): e13004. doi: 10.1111/acel.13004 [24] Jeong M H, Kim H J, Pyun J H, et al. Cdon deficiency causes cardiac remodeling through hyperactivation of WNT/beta-catenin signaling. Proc Natl Acad Sci U S A, 2017(8); 114: E1345-E1354. [25] Kakugawa S, Langton PF, Zebisch M, et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature, 2015; 519(7542): 187-192. doi: 10.1038/nature14259 [26] Gerhardt B, Leesman L, Burra K, et al. Notum attenuates Wnt/ β-catenin signaling to promote tracheal cartilage patterning. Dev Biol, 2018; 436(1): 14-27. doi: 10.1016/j.ydbio.2018.02.002 [27] Cai B, Tan X, Zhang Y, et al. Mesenchymal stem cells and cardiomyocytes interplay to prevent myocardial hypertrophy. Stem Cells Transl Med, 2015; 4(12): 1425-1435. doi: 10.5966/sctm.2015-0032 [28] Ma H, Killaars A R, DelRio F W, et al. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials, 2017; 131: 131-144. doi: 10.1016/j.biomaterials.2017.03.040 [29] Sapudom J, Rubner S, Martin S, et al. The interplay of fibronectin functionalization and TGF-beta1 presence on fibroblast proliferation, differentiation and migration in 3D matrices. Biomater Sci, 2015; 3(9): 1291-301. doi: 10.1039/C5BM00140D [30] Nagaraju C K, Robinson E L, Abdesselem M, et al. Myofibroblast phenotype and reversibility of fibrosis in patients with end-stage heart failure. J Am Coll Cardiol, 2019; 73(18): 2267-2282. doi: 10.1016/j.jacc.2019.02.049 [31] Voss A K, Krebs D L, Thomas T. C3G regulates the size of the cerebral cortex neural precursor population. EMBO J, 2006; 25(15): 3652-3663. doi: 10.1038/sj.emboj.7601234 [32] Detienne G, De Haes W, Mergan L, et al. Beyond ROS clearance: Peroxiredoxins in stress signaling and aging. Ageing Res Rev, 2018; 44: 33-48. doi: 10.1016/j.arr.2018.03.005 [33] Gourdie R G, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov, 2016; 15(9): 620-638. doi: 10.1038/nrd.2016.89 [34] Kologrivova I, Shtatolkina M, Suslova T, et al. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol, 2021; 12: 664457. doi: 10.3389/fimmu.2021.664457 -


 投稿系统
投稿系统


 下载:
下载: