-
Abstract:
Objective Clinical manifestation of the inflammatory process in its relation to biochemical markers (total cysteine [Cys], cysteine-glycine [CysGly], glutathione [GSH], glutamate-cysteine [Glu-Cys], homocysteine [Hcy], the ratio of reduced to oxidized glutathione [GSH/GSSG], the ratio of reduced to oxidized cysteine [CySH/CySS], malondialdehyde-oxidized low-density lipoproteins [MDA-oxLDL]) has been studied in patients with coronavirus disease 2019 (COVID-19). Material and methods 48 patients with mild to severe COVID-19 and 20 healthy volunteers were included in our research. The participants were divided into 4 experimental groups according to inflammation intensity estimated based on the serum levels of interleukin 6 (IL-6). Results All 4 groups showed the prevalence of male patients and elevated serum levels of IL-6 (by 54.6%). There was no comorbidity in patients with mild COVID-19 (nasopharyngitis symptoms) and in healthy control subjects. 50% of patients with lung damage had accompanying diseases. Alterations of aminoethyl metabolism were detected in COVID-19 patients: as reflected by the decreased levels of Cys, CysGly, and Glu-Cys and the increased levels of GSH as compared to the control group. Conclusion Elevation of IL-6 over 7.5 pg/mL was associated with decreased GSH/GSSG and CySH/CySS ratios indicating enhanced oxidative stress and was followed by protein oxidation, specifically MDA-oxLDL. -
Key words:
- coronavirus disease 2019 /
- aminothiols /
- oxidative stress /
- interleukin-6 /
- oxidized lipoproteins
-
Table 1. Baseline characteristics of the patients with COVID-19
Indicator 1st subgroup
(N = 20)2nd subgroup
(N = 10)3rd subgroup
(N = 26)4th subgroup
(N = 12)Kruskal-Wallis test Interleukin 6, pg/mL 2.25
(1.11, 3.55)8.96
(7.17, 12.86)15.48
(13.25, 23.46)b44.98
(35.50, 65.91)a, cχ2 = 16.98
Р = 0.000 01Gender (female/male), N 10/10 2/8 8/18 5/7 - Age, years 43.00
(30.65, 50.20)31.00
(27.75, 41.00)46.00
(33.75, 52.20)49.00
(44.00, 50.60)χ2 = 5.38
Р = 0.15Chest computed tomography severity score 0
(0, 0)0
(0, 0)2 (1, 2)a 3 (1, 4)a - Body temperature, ℃ 36.6
(35.5, 36.8)36.8
(36.6, 37.65)a38.5
(37.68, 38.62)a, b38.6
(37.80, 38.92)a, bχ2 = 13.20
Р = 0.004Blood oxygen Saturation, % 99.80
(98.50, 99.90)97.00
(96.25, 97.75)a95.50
(93.75, 97.20)a, b94.00
(93.00, 96.40)aχ2 = 14.54
Р = 0.002MDA-oxLDL 0
(0, 0.12)8.66
(3.78, 11.60)a1.81
(0.50, 7.50)a0.49
(0.34, 2.87)aχ2 = 15.34
Р = 0.002Systolic blood pressure, mm Hg 120
(120, 130)130
(115, 130)130
(120, 145)150
(150, 155) a, cχ2 = 13.48
Р = 0.004Diastolic blood pressure, mm Hg 80
(75, 85)80
(75, 85)90
(80, 95)90
(90, 95) a, bχ2 = 13.03
Р = 0.005Atherosclerosis, % - - 20 17 - Arterial hypertension, % - - 10 20 - N, the number of patients; a, P < 0.05, vs. control group; b, P < 0.05, the 1st subgroup vs. the 2nd and the 3rd subgroups; c, P < 0.05, the 3rd subgroup vs. the 4th group; COVID-19, coronavirus disease 2019. Table 2. Blood serum level of aminothiols in COVID-19 patients
Indicator 1st subgroup
(N = 20)2nd subgroup
(N = 10)3rd subgroup
(N = 26)4th subgroup
(N = 12)Kruskal–Wallis test Cys, µmol/L 212.60
(167.70, 264.80)207.67
(200.21, 235.45)a294.00
(271.72, 357.35)173.24
(146.51, 329.17)aχ2 = 10.49
Р = 0.01CysGly, µmol/L 43.20
(39.50-53.90)18.39
(13.74, 36.92)a17.37
(14.59, 33.37)a15.60
(10.43, 44.68)aχ2 = 9.57
Р = 0.04GSH, µmol/L 3.10
(2.60, 3.80)3.25
(2.49, 4.35)5.60
(4.71, 6.21)a7.85
(7.20, 9.24)a, cχ2 = 13.93
Р = 0.003Glu-Cys, µmol/L 3.20 (2.40-3.90) 6.99
(5.64, 7.84)a5.50
(5.12, 6.48)a3.89
(3.51, 8.00)cχ2 = 12.21
Р = 0.007Hcy, µmol/L 6.75
(5.77, 7.55)9.97
(9.31, 10.22)a16.18
(13.28, 21.55)a, b15.94
(15.27, 19.61)aχ2 = 16.36
Р = 0.000 01GSH/GSSG 9.00
(8.97, 9.18)0.10
(0.05, 0.19)a0.23
(0.14, 0.37)a0.25
(0.17, 0.27)aχ2 = 12.08
Р = 0.004CySH/CySS 1.67
(1.02, 1.88)1.35
(1.22, 1.56)0.59
(0.37, 0.69)0.14
(0.06, 0.34)χ2 = 15.06
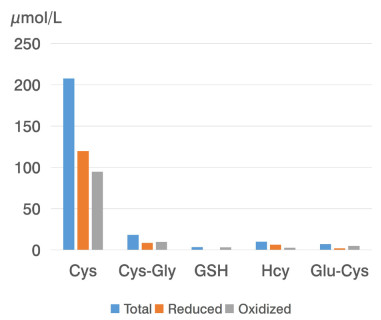
Р = 0.002N, the number of patients; a, P < 0.05, vs. control group; b, P < 0.05, the 1st subgroup vs. the 2nd and the 3rd subgroups; c, P < 0.05, the 3rd subgroup vs. the 4th group; Cys, cysteine; CysGly, cysteine-glycine; GSH, glutathione; Hcy, homocysteine; Glu-Cys, glutamate-cysteine; COVID-19, coronavirus disease 2019. -
[1] Checconi P, DeAngelis M, Marcocci M E, et al. Redox-modulating agents in the treatment of viral infections. Int J Mol Sci, 2020; 21(11): 4084. doi: 10.3390/ijms21114084 [2] Lavillette D, Barbouche R, Yao Y, et al. Significant redox insensitivity of the functions of the SARS-CoV spike glycoprotein: comparison with HIV envelope. J Biol Chem, 2006; 281(14): 9200-9204 doi: 10.1074/jbc.M512529200 [3] Fenouillet E, Barbouche R, Jones I M. Cell entry by enveloped viruses: redox considerations for HIV and SARS-coronavirus. Antioxid Redox Signal, 2007; 9(8): 1009-1034. doi: 10.1089/ars.2007.1639 [4] Kryukov E V, Ivanov A V, Karpov V O, et al. Association of low molecular weight plasma aminothiols with the severity of Coronavirus disease 2019. Oxid Med Cell Longev, 2021; 2021: 9221693. [5] Musaogullari A, Chai Y C. Redox regulation by protein S-glutathionylation: from molecular mechanisms to implications in health and disease. Int J Mol Sci, 2020; 21(21): 8113. doi: 10.3390/ijms21218113 [6] Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J Gen Med, 2011; 4: 105-113. [7] Rochette L, Ghibu S. Mechanics insights of alpha-lipoic acid against cardiovascular diseases during COVID-19 infection. Int J Mol Sci, 2021; 22(15): 7979. doi: 10.3390/ijms22157979 [8] Focks J J, Van Schaik A., Clappers N, et al. Assessment of plasma aminothiol levels and the association with recurrent atherothrombotic events in patients hospitalized for an acute coronary syndrome: a prospective study. Clin Chem Lab Med, 2013; 51(11): 2187-2193. doi: 10.1515/cclm-2013-0103 [9] Fefelova E V, Tereshkov P P, Maksimenya M V, et al. Human lymphocytes develop apoptosis when exposed with aminothyols in short-term cell culture. Transbaikalian Med Bulletin, 2016; 2: 98-106. [10] The prevention, diagnosis and treatment of the new coronavirus infection 2019-nCoV. Temporary guidelines Ministry of Health of the Russian Federation. Pulmonologiya, 2019; 29(6): 655-672. [11] Elesela S, Lukacs NW. Role of mitochondria in viral infections. Life, 2021; 11: 232. doi: 10.3390/life11030232 [12] Alekseeva E I, Tepaev R F, Shilkrot I, et al. COVID-19-associated secondary hemophagocytic lymphohistiocytosis (the syndrome of "cytokine storm"). Annals of the Russian Academy of Medical Sciences, 2021; 76(1): 51-66. doi: 10.15690/vramn1410 [13] Khomich O A, Kochetkov S N, Bartosch B, et al. Redox biology of respiratory viral infections. Viruses, 2018; 10(8): 392. doi: 10.3390/v10080392 [14] Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin Ⅱ -mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest, 1996; 97: 1916-1923. doi: 10.1172/JCI118623 [15] Voronina T A. Antioxidants/antihypoxants: the missing puzzle piece in effective pathogenetic therapy for COVID-19. Infektsionnye bolezni, 2020; 2: 97-102. [16] Mittal M, Siddiqui M, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal, 2014; 20(7): 1126- 1167. doi: 10.1089/ars.2012.5149 [17] Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. status of the art. Clin Interv Aging, 2018; 13: 757-772. doi: 10.2147/CIA.S158513 [18] Bin P, Huang R, Zhou X. Oxidation resistance of the sulfur amino acids: methionine and cysteine. Biomed Res Int, 2017; 2017: 9584932. [19] Suhai S, Zajac J, Fossum C, et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (Covid-19) infection: a review. Protein J, 2020; 26: 1-13. [20] Bartolini D, Wang Y, Zhang J, et al. Selenohormetine protects bone marrow hematopoietic cells against ionizing radiation-induced toxicities. PLoS ONE, 2019; 14(4): e0205626. [21] Singh J, Dhindsa R S, Misra V, et al. SARS-CoV2 infectivity is potentially modulated by host redox status. Comput Struct Biotechnol J, 2020; 18: 3705-3711. -


 投稿系统
投稿系统


 下载:
下载: