Cryptotanshinone increases the sensitivity of liver cancer to sorafenib by inhibiting the STAT3/Snail/epithelial mesenchymal transition pathway
doi: 10.2478/fzm-2022-0016
-
Abstract:
Objective Sorafenib resistance has been a major factor limiting its clinical use as a targeted drug in liver cancer. The present study aimed to investigate whether cryptotanshinone can enhance the sensitivity of liver cancer and reduce the resistance to sorafenib. Methods Sorafenib-resistant cells were established based on HepG2 and Huh7 cell lines. And the anti-tumor effect of sorafenib combined with cryptotanshinone on the sorafenib-resistant cells was verified by MTT, colony formation, transwell assays and tumor growth xenograft model. Moreover, the effects of the combined treatment on the expression of phosphorylated (p)-STAT3, as well as epithelial mesenchymal transition (EMT) and apoptosis related proteins of cells were evaluated by western blot analysis. Results It was identified that cryptotanshinone inhibited the viability of both HepG2 and Huh7 cells in a dose- and time-dependent manner, and decreased p-STAT3 expression rather than total STAT3 expression at a concentration of 40 njmol/L. In the sorafenib-resistant cells, sorafenib in combination with cryptotanshinone markedly inhibited cell viability, invasion and migration compared with sorafenib alone. In contrast, increased p-STAT3 level by colivelin led to the inhibition of the synergistic effect of cryptotanshinone and sorafenib not only on cell viability, but also on EMT and apoptosis, suggesting that cryptotanshinone and sorafenib may act by downregulating STAT3 signaling. Further, the inhibition of carcinogenicity effect was also verified in xenografted tumor models. Conclusion The present results indicated that cryptotanshinone could synergize with sorafenib to inhibit the proliferative, invasive, and migratory abilities of sorafenib-resistant cells by downregulating STAT3 signaling. -
Key words:
- cryptotanshinone /
- sorafenib /
- sorafenib-resistance /
- STAT3 signaling /
- liver cancer
-
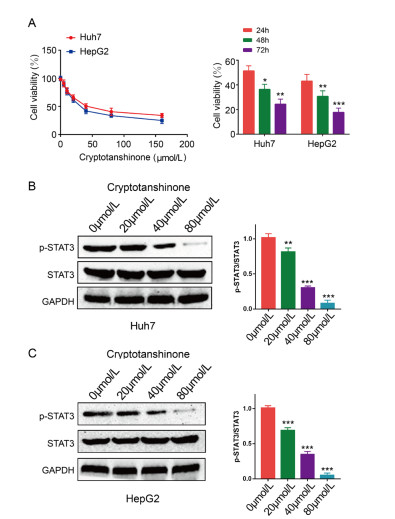
Figure 1. Cryptotanshinone inhibits the proliferation and STAT3 activation of liver cancer cells.
(A) HepG2 and Huh7 cells were exposed to different concentrations (0, 5, 10, 20, 40, 80, 160 and 320 μmol/L) of cryptotanshinone for 24, 48 and 72 h. (B, C) The expression levels of p-STAT3 and STAT3 were measured via western blotting. The band density of p-STAT3 was normalized to STAT3. The data are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2. Sorafenib inhibits STAT3 activation in sorafenib-sensitive cells but not in sorafenib-resistant liver cancer cells.
The expression levels of p-STAT3 and STAT3 were measured by western blot analysis. (A) Sorafenib-resistant cells and parental cells were incubated with or without 10 μmol/L sorafenib for 24 h. (B, C) Sorafenib-resistant cells and parental cells were incubated with sorafenib at serial concentrations of 0, 2, 5 or 10 μmol/L for 24 h. The protein levels were detected via western blot analysis. GAPDH served as the loading control. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3. Cryptotanshinone synergizes with sorafenib to inhibit the malignant biological behavior of sorafenib-resistant cells.
(A) HepG2-SR and Huh7-SR cells were exposed to different concentrations (0, 20, 40 and 80 μmol/L) of cryptotanshinone and/or different concentrations (0, 2, 5 and 10 μmol/L) of sorafenib for 24 h. Cell viability was assessed and normalized to the values from control cells. (B) Huh7-SR and HepG2-SR cells were incubated for 48 h with sorafenib (10 μmol/L), cryptotanshinone (40 μmol/L) or a combination. Cell viability was assessed and normalized to control cells. ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Cryptotanshinone; & & & P < 0.001 vs. Sorafenib alone. (C, D) Cells described in panel (A) were subjected to Transwell assay for 48 h, and cell migration and invasion were evaluated.***P < 0.001 vs. Control; #P < 0.05, ###P < 0.001 vs. Cryptotanshinone; & & & P < 0.001 vs. Sorafenib alone.
Figure 4. Cryptotanshinone synergizes with sorafenib to inhibit p-STAT3, EMT and promote apoptosis in sorafenib-resistant cells.
Western blot analysis of protein levels of p-STAT3 (A), and EMT-(B) and apoptosis-associated markers (C) in Huh7-SR and HepG2-SR. Band densities were normalized to GAPDH. Data represent three independent experiments. **P < 0.01, ***P < 0.001 vs. Control; ##P < 0.01, ###P < 0.001 vs. Cryptotanshinone; & & & P < 0.001 vs. Sorafenib alone.
Figure 5. Activation of STAT3 enhances the malignant biological behavior of sorafenib-resistant cells.
(A) HepG2-SR and Huh7-SR cells were incubated with 0 or 10 μmol/L colivelin for 0.5 h, and then incubated with 40 μmol/L cryptotanshinone and/or 10 μmol/L sorafenib for 24 h. Cell viability was assessed and compared with the corresponding untreated cells. *P < 0.05, ***P < 0.001. (B) Cells were subjected to colony formaiton assay. *P < 0.05 vs. Control; #P < 0.05 vs. Cryptotanshinone; & P < 0.05 vs. Sorafenib; %P < 0.05 vs. combination. (C, D) The cells were subjected to Transwell assay for 48 h, and then cell migration and invasion were measured. *P < 0.05, ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01 vs. Cryptotanshinone; & P < 0.05, & & & P < 0.001 vs. Sorafenib; %P < 0.05, %%P < 0.01, %%%P < 0.001 vs. combination.
Figure 6. Activation of STAT3 enhances EMT activation and inhibits apoptosis of the sorafenib-resistant liver cancer cells.
Western blot analysis of the protein levels of p-STAT3 (A) and EMT-(B) and apoptosis-associated markers (C) in Huh7-SR and HepG2-SR. Band densities were normalized to GAPDH. Data represent three independent experiments. ***P < 0.001.
Figure 7. Cryptotanshinone increases the sensitivity to sorafenib in sorafenib-resistant cells in vivo.
(A) Xenograft tumor images from different groups (N = 3). (B) Measurement of tumor volume (N = 3). (C) Measurement of the tumor weight of different groups (N = 3). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. Cryptotanshinone; & P < 0.05, & & P < 0.01 vs. Sorafenib alone.
-
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018; 68(6): 394-424. doi: 10.3322/caac.21492 [2] Rastogi S, Haldar C. Role of melatonin and HSF-1\HSP-70 in modulating cold stress-induced immunosuppression in a tropical rodentFunambulus pennanti[Volume 87, January 2020, 102456]. J Therm Biol, 2021; 98: 102941. doi: 10.1016/j.jtherbio.2021.102941 [3] Cheng A L, Kang Y K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase Ⅲ randomised, double-blind, placebo-controlled trial. Lancet Oncol, 2009; 10(1): 25-34. doi: 10.1016/S1470-2045(08)70285-7 [4] Keating G M. Sorafenib: A Review in Hepatocellular Carcinoma. Target Oncol, 2017; 12(2): 243-253. doi: 10.1007/s11523-017-0484-7 [5] Subramaniam A, Shanmugam M K, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta, 2013; 1835(1): 46-60. [6] Pan X, Feng J, Zhu Z, et al. A positive feedback loop between miR-181b and STAT3 that affects Warburg effect in colon cancer via regulating PIAS3 expression. J Cell Mol Med, 2018; 22(10): 5040-5049. doi: 10.1111/jcmm.13786 [7] Van Malenstein H, Dekervel J, Verslype C, et al. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-tomesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett, 2013; 329(1): 74-83. doi: 10.1016/j.canlet.2012.10.021 [8] Xie L, Zeng Y, Dai Z, et al. Chemical and genetic inhibition of STAT3 sensitizes hepatocellular carcinoma cells to sorafenib induced cell death. Int J Biol Sci, 2018; 14(5): 577-585. doi: 10.7150/ijbs.22220 [9] Tse A K, Chow K Y, Cao H H, et al. The herbal compound cryptotanshinone restores sensitivity in cancer cells that are resistant to the tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem, 2013; 288(41): 29923-29933. doi: 10.1074/jbc.M113.483909 [10] Lu L, Zhang S, Li C, et al. Cryptotanshinone inhibits human glioma cell proliferation in vitro and in vivo through SHP-2-dependent inhibition of STAT3 activation. Cell Death Dis, 2017; 8(5): e2767. doi: 10.1038/cddis.2017.174 [11] Shin D S, Kim H N, Shin K D, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res, 2009; 69(1): 193-202. doi: 10.1158/0008-5472.CAN-08-2575 [12] Ji Y, Liu Y, Xue N, et al. Cryptotanshinone inhibits esophageal squamous-cell carcinoma in vitro and in vivo through the suppression of STAT3 activation. OncoTargets Ther, 2019; 12: 883-896. doi: 10.2147/OTT.S187777 [13] Yen J H, Huang H S, Chuang C J, et al. Activation of dynaminrelated protein 1-dependent mitochondria fragmentation and suppression of osteosarcoma by cryptotanshinone. J Exp Clin Cancer Res, 2019; 38(1): 42. doi: 10.1186/s13046-018-1008-8 [14] Park I J, Yang W K, Nam S H, et al. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis, 2014; 19(4): 615-628. doi: 10.1007/s10495-013-0929-0 [15] Shen L, Zhang G, Lou Z, et al. Cryptotanshinone enhances the effect of Arsenic trioxide in treating liver cancer cell by inducing apoptosis through downregulating phosphorylated-STAT3 in vitro and in vivo. BMC Complement Altern Med, 2017; 17(1): 106. doi: 10.1186/s12906-016-1548-4 [16] Polyak K, Weinberg R A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer, 2009; 9(4): 265-273. doi: 10.1038/nrc2620 [17] Ombrato L, Malanchi I. The EMT universe: space between cancer cell dissemination and metastasis initiation. Crit Rev Oncog, 2014; 19(5): 349-361. doi: 10.1615/CritRevOncog.2014011802 [18] Dong J, Zhai B, Sun W, et al. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelialmesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PloS One, 2017; 12(9): e0185088. doi: 10.1371/journal.pone.0185088 [19] Liu K, Tian T, Zheng Y, et al. Scutellarin inhibits proliferation and invasion of hepatocellular carcinoma cells via down-regulation of JAK2/STAT3 pathway. J Cell Mol Med, 2019; 23(4): 3040-3044. doi: 10.1111/jcmm.14169 [20] Xiong H, Hong J, Du W, et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelialmesenchymal transition. J Biol Chem, 2012; 287(8): 5819-5832. doi: 10.1074/jbc.M111.295964 [21] Zhai B, Hu F, Yan H, et al. Bufalin Reverses Resistance to Sorafenib by Inhibiting Akt Activation in Hepatocellular Carcinoma: The Role of Endoplasmic Reticulum Stress. PloS One, 2015; 10(9): e0138485. doi: 10.1371/journal.pone.0138485 [22] Ou D L, Shyue S K, Lin L I, et al. Growth arrest DNA damageinducible gene 45 gamma expression as a prognostic and predictive biomarker in hepatocellular carcinoma. Oncotarget, 2015; 6(29): 27953- 27965. doi: 10.18632/oncotarget.4446 [23] Yin X, Zhang B H, Zheng S S, et al. Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/ Snail signaling. J Hematol Oncol, 2015; 8: 23. doi: 10.1186/s13045-015-0119-3 [24] Liu Y, Liu L, Zhou Y, et al. CKLF1 Enhances Inflammation-Mediated Carcinogenesis and Prevents Doxorubicin-Induced Apoptosis via IL6/ STAT3 Signaling in HCC. Clin Cancer Res, 2019; 25(13): 4141-4154. doi: 10.1158/1078-0432.CCR-18-3510 [25] Xu J, Zheng L, Chen J, et al. Increasing AR by HIF-2α inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis, 2017; 8(10): e3095. doi: 10.1038/cddis.2017.411 [26] Huang C Y, Lin C S, Tai W T, et al. Sorafenib enhances radiationinduced apoptosis in hepatocellular carcinoma by inhibiting STAT3. Int J Radiat Oncol Biol Phys, 2013; 86(3): 456-462. doi: 10.1016/j.ijrobp.2013.01.025 [27] Li J, Zhou Y, Liu Y, et al. Sorafenib inhibits caspase-1 expression through suppressing TLR4/stat3/SUMO1 pathway in hepatocellular carcinoma. Cancer Biol Ther, 2018; 19(11): 1057-1064. doi: 10.1080/15384047.2018.1480280 [28] Su J C, Tseng P H, Wu S H, et al. SC-2001 overcomes STAT3- mediated sorafenib resistance through RFX-1/SHP-1 activation in hepatocellular carcinoma. Neoplasia (New York, NY), 2014; 16(7): 595- 605. doi: 10.1016/j.neo.2014.06.005 [29] Gong C, Zhang Y, Shankaran H, et al. Integrated analysis reveals that STAT3 is central to the crosstalk between HER/ErbB receptor signaling pathways in human mammary epithelial cells. Mol BioSyst, 2015; 11(1): 146-158. doi: 10.1039/C4MB00471J [30] Morales-Prieto D M, Ospina-Prieto S, Chaiwangyen W, et al. Intranuclear crosstalk between extracellular regulated kinase1/2 and signal transducer and activator of transcription 3 regulates JEG-3 choriocarcinoma cell invasion and proliferation. ScientificWorldJournal, 2013; 2013: 259845. [31] Chen K F, Tai W T, Hsu C Y, et al. Blockade of STAT3 activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur J Med Chem, 2012; 55: 220-227. doi: 10.1016/j.ejmech.2012.07.023 [32] Ke F, Wang Z, Song X, et al. Cryptotanshinone induces cell cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFκB pathways in cholangiocarcinoma cells. Drug Des Devel Ther, 2017; 11: 1753-1766. doi: 10.2147/DDDT.S132488 [33] Dong B, Liang Z, Chen Z, et al. Cryptotanshinone suppresses key onco-proliferative and drug-resistant pathways of chronic myeloid leukemia by targeting STAT5 and STAT3 phosphorylation. Sci China Life Sci, 2018; 61(9): 999-1009. doi: 10.1007/s11427-018-9324-y [34] Lai S C, Su Y T, Chi C C, et al. DNMT3b/OCT4 expression confers sorafenib resistance and poor prognosis of hepatocellular carcinoma through IL-6/STAT3 regulation. J Exp Clin Cancer Res, 2019; 38(1): 474. doi: 10.1186/s13046-019-1442-2 -


 投稿系统
投稿系统


 下载:
下载: