Nortriterpenoids from the fruit stalk of Schisandra chinensis (Turcz.) Baill.
doi: 10.2478/fzm-2022-0014
-
Abstract:
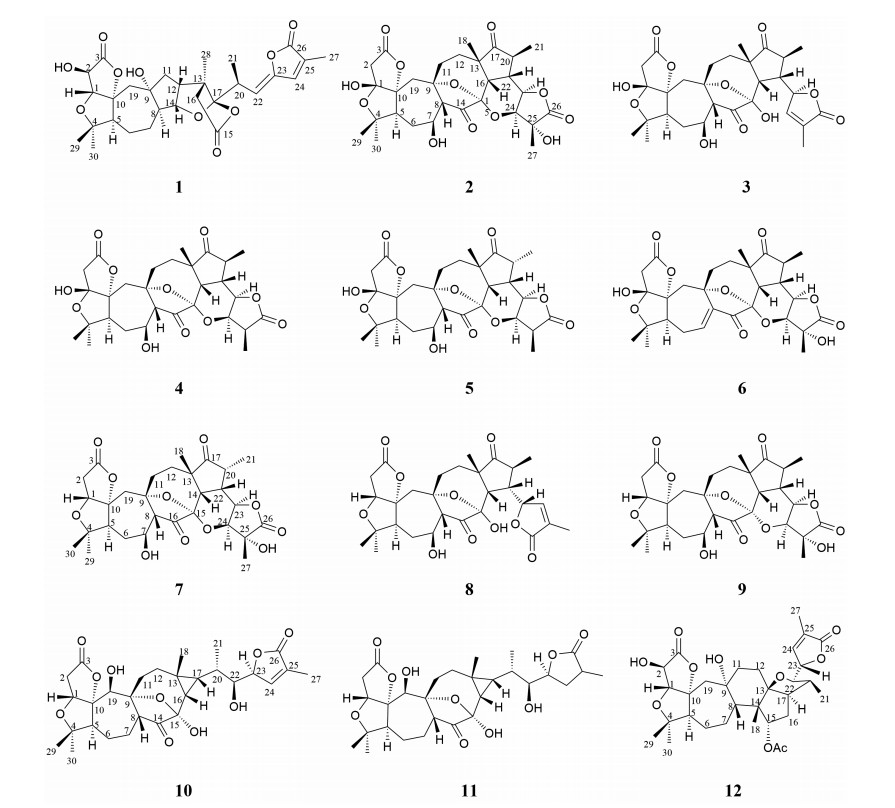
Objective The fruit stalk of Schisandra chinensis (Turcz.) Baill. (S. chinensis) has been found to contain bioactive components similar to the fruit of S. chinensis. Here, we report a recent discovery about new nortriterpenoids with a novel skeleton and anti-gastric cancer activity, which were isolated from the fruit stalk of S. chinensis. Methods The chemical components of ethyl acetate extract from 70% ethanol extract from S. chinensis fruit stalk were separated, purified, and identified by liquid chromatography methods (silica gel, ODS, HPLC) and extensive spectroscopic analyses (NMR, IR, UV, MS, CD). Results Two new nortriterpenoids, schilancitrilactone M and 25-hydroxyl schindilactone D (1 and 2), along with ten known nortriterpenoids (3-12) were isolated from the fruit stalk of S. chinensis. The isolated compounds were tested for their cytotoxic activities against MGC-803 cells, and the results showed that compounds 6-8 possessed significant activities with IC50 of 9.01, 11.77, and 2.74 μmol/L, respectively. Conclusion Twelve nortriterpenoids including two new compounds were isolated from the fruit stalk of S. chinensis for the first time. Among them, compounds 6-8 showed significant anti-gastric cancer activities. We postulated that the fruit stalk of S. chinensis could be used as an anti-gastric cancer drug. -
Table 1. 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectroscopic data for 1 (δ in ppm, J in Hz) in C5D5N
No δC δH(J in Hz) 1 87.1 4.42 (1H, s) 2 73.0 4.72 (1H, s) 3 176.6 4 85.3 5 60.5 2.44 (1H, dd, J=4.6, 12.2) 6 22.1 1.36-1.42 (2H, m) 7 24.9 1.95 (2H, m) 8 54.5 2.65(1H, m) 9 82.8 10 99.3 11 43.8 2.13 (1H, o) 1.75 (1H, dd, J=8.4, 12.6) 12 50.9 2.70 (1H, o) 13 50.7 14 85.5 4.75 (1H, dd, J=6.0, 8.0) 15 173.5 16 46.3 2.85 (2H, o) 17 121.9 19 42.5 2.82 (1H, ABd, J=15.6) 2.37 (1H, ABd, J=15.6) 20 36.5 3.52 (1H, m) 21 16.3 1.31 (3H, d, J=6.8) 22 114.3 5.59 (1H, d, J=9.8) 23 148.2 24 138.5 6.98 (1H, s) 25 129.6 26 170.8 6.98 27 10.4 1.83 (3H, s) 28 19.0 1.14 (3H, s) 29 22.2 1.03 (3H, s) 30 28.8 1.24 (3H, s) Table 2. 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectroscopic data for 2 (δ in ppm, J in Hz) in C5D5N
No δC δH(J in Hz) 1 108.7 2 43.5 3.04(1H, ABd, J=17.8) 3.13 (1H, ABd, J=17.8) 3 173.4 4 84.2 5 58.9 2.64 (1H, m) 6 36.4 2.26 (2H, m) 7 68.4 4.60 (1H, m) 8 60.2 2.93 (1H, d, J=9.8) 9 81.7 10 97.0 11 42.4 1.73 (1H, m) 2.08 (1H, m) 12 31.2 1.59 (1H, m) 1.84 (1H, m) 13 50.2 14 209.8 15 99.1 16 45.0 2.88 (1H, o) 17 220.4 18 26.0 0.93 (3H, s) 19 40.7 2.61 (1H, ABd, 16.3) 2.83 (1H, ABd, 16.3) 20 44.6 2.75 (1H, dq, J=7.0, 12, 0) 21 14.9 1.05 (3H, d, J=7.0) 22 40.3 2.88 (1H, m) 23 75.1 5.30 (1H, br. s) 24 73.3 5.22 (1H, d, J=1.8) 25 76.7 26 177.7 27 17.6 1.71 (3H, s) 29 25.1 1.39 (3H, s) 30 29.5 1.26 (3H, s) Table 3. Cytotoxic activities of compounds 1-12 against MGC-803 cell lines
Drugs IC50(µmol/L) compound 1 46.38 ± 3.92 compound 2 ﹥50 compound 3 ﹥50 compound 4 ﹥50 compound 5 ﹥50 compound 6 9.01 ± 3.72 compound 7 11.77 ± 2.61 compound 8 2.74 ± 0.58 compound 9 ﹥50 compound 10 20.17 ± 3.12 compound 11 40.85 ± 3.65 compound 12 ﹥50 cisplatin 3.79 ± 0.51 IC50 was defined as the concentration that resulted in a 50% decrease in cell number, and the values were within the 0–1 000 μg/mL range. Results are presented as mean ± SD (N = 3 independent replicates).
Cisplatin was used as a positive control.S1. 13C-NMR (100 MHz) spectroscopic data for compounds 3-12.
No. 3 4 5 6 7 8 9 10 11 12 1 109.0 108.7 108.8 108.2 81.6 81.7 81.4 79.8 79.5 87.3 2 43.1 43.6 43.1 44.0 35.1 35.5 35.4 35.9 35.4 73.7 3 173.7 173.6 173.4 173.2 175.2 174.6 175.5 176.0 175.5 177.2 4 84.6 84.1 84.6 83.8 84.0 83.9 83.8 84.6 84.2 85.8 5 58.0 58.9 58.3 58.5 58.2 58.3 58.4 62.4 62.2 58.7 6 37.0 36.3 36.6 23.6 36.6 36.9 36.4 24.3 23.9 29.3 7 69.9 68.2 68.8 135.2 68.0 68.8 67.8 27.6 27.2 25.8 8 59.6 60.2 60.5 138.0 60.6 59.7 60.2 56.9 56.6 57.6 9 83.6 81.6 81.7 82.0 81.5 79.7 81.5 83.1 82.6 70.6 10 97.5 97.0 97.4 96.4 95.9 96.1 95.7 99.0 98.4 100.6 11 43.4 42.4 41.5 39.6 41.0 42.9 41.8 39.1 38.6 38.0 12 32.8 31.2 30.4 31.4 30.3 32.5 31.0 26.3 25.8 34.6 13 50.4 50.2 49.9 50.6 49.8 50.4 50.2 27.0 26.5 93.0 14 215.1 209.9 209.5 198.8 45.6 53.7 45.0 216.8 216.5 53.4 15 101.2 98.8 98.1 99.3 98.3 101.1 99.1 99.9 99.4 81.9 16 53.7 45.1 45.8 45.6 209.1 214.7 209.5 31.4 30.9 30.7 17 220.0 220.4 221.6 220.3 221.6 221.9 220.4 35.6 35.2 53.0 18 28.0 26.0 26.0 26.4 25.9 28.0 26.0 29.2 28.8 24.0 19 40.2 40.6 40.8 40.7 42.6 41.8 42.6 71.6 70.9 49.8 20 46.3 44.8 33.2 44.6 41.2 46.1 40.2 33.3 31.8 35.9 21 15.6 15.0 12.4 14.9 12.4 15.6 14.8 20.0 19.5 12.0 22 46.3 40.3 41.2 40.3 33.2 46.3 44.6 76.0 78.9 87.0 23 80.1 75.5 75.0 74.9 74.6 83.6 73.2 84.0 79.1 81.2 24 151.3 69.4 71.7 72.6 75.6 151.4 75.0 149.2 34.0 147.1 25 131.5 42.1 42.2 77.0 76.9 131.5 76.7 130.8 34.8 130.4 26 174.6 178.0 177.8 177.7 177.2 175.9 177.5 175.7 181.4 174.4 27 10.8 8.0 7.9 18.1 17.5 10.9 17.5 11.2 16.6 10.8 29 25.4 25.0 25.4 24.7 27.8 27.9 27.8 22.4 22.0 24.3 30 30.0 29.4 29.8 29.0 21.0 21.0 20.9 29.0 28.6 30.1 OAC - - - - - - - - - 169.9 - - - - - - - - - - 21.6 -
[1] Szopa A, Ekiert R, Ekiert H. Current knowledge of Schisandra chinensis (Turcz. ) Baill. (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studie. Phytochem Rev, 2017; 16: 195-218. [2] Jin M H, Liu R J. Analysis of content of the Schisandra chinensis Baill fruit, rattan and fruit handles. J Med Sci, 2005; 1: 28-30. [3] Jin Y P, Yan S, Liu J X, et al. Reseach progress on nortriterpenoids in plants of Schisandraceae and their pharmacological activities. Zhong Cao Yao, 2014; 45(11): 1643-1650. [4] Yan B C, Hu K, Sun H D, et al. Recent advances in the synthesis of isodon diterpenoids and schinortriterpenoids. Chinese J Org Chem, 2018; 38: 2259-2280. doi: 10.6023/cjoc201806002 [5] Luo X, Shi Y M, Luo R H, et al. Schilancitrilactones A-C: three unique nortriterpenoids from Schisandra lancifolia. Org Lett, 2012; 14(5): 1286-1289. doi: 10.1021/ol300099e [6] Huang S X, Yang L B, Xiao W L, et al. Wuweizidilactones A-F: Novel highly oxygenated nortriterpenoids with unusual skeletons isolated from Schisandra chinensis. Chem Eur J, 2007; 13(17): 4816-4822. doi: 10.1002/chem.200700346 [7] Shi Y M, Hu K, Pescitelli G, et al. Schinortriterpenoids with identical configuration but distinct ECD spectra generated by nondegenerate exciton coupling. Org Lett, 2018; 20(6): 1500-1504. doi: 10.1021/acs.orglett.8b00149 [8] Xiao W L, Huang S X, Zhang L, et al. Nortriterpenoids from Schisandra lancifolia. J Nat Prod, 2006; 69(2): 650-653. [9] Huang S X, Han Q B, Lei C, et al. Isolation and characterization of miscellaneous terpenoids of Schisandra chinensis. Cheminform, 2008; 64(64): 4260-4267. [10] Huang S X, Li R T, Liu J P, et al. Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Cheminform, 2007; 9(11): 2079-2082. [11] Xue Y B, Zhang Y L, Yang J H, et al. Nortriterpenoids and lignans from the fruit of Schisandra chinensis. Chem Pharm Bull, 2010; 58(12): 1606-1611. doi: 10.1248/cpb.58.1606 [12] Li R T, Xiang W, Li S H, et al. Lancifodilactones B-E, new nortriterpenes from Schisandra lancifolia. J Nat Prod, 2004; 67(1): 94-97. doi: 10.1021/np030339+ [13] Shi Y M, Wang X B, Li X N, et al. Lancolides, antiplatelet aggregation nortriterpenoids with tricyclo[6.3. 0.02, 11] undecane-bridged system from Schisandra lancifolia. Org Lett, 2013; 15(19): 5068-5071. doi: 10.1021/ol402414z [14] Cheng Y B, Liao T C, Lo Y W, et al. Nortriterpene lactones from the fruits of Schisandra arisanensis. J Nat Prod, 2010; 73(7): 1228-1233. doi: 10.1021/np100048h -


 投稿系统
投稿系统


 下载:
下载: