Challenges and improvement in management of neonates born to mothers with COVID-19 in China
doi: 10.2478/fzm-2022-0013
-
Abstract:
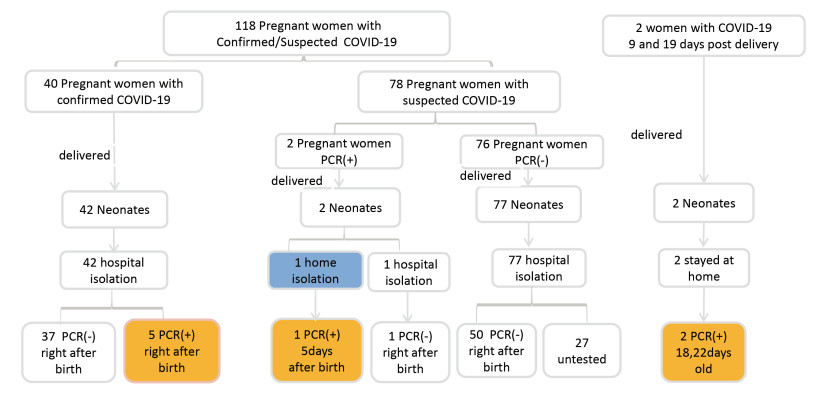
Objective China was the first country suffering from the SARS-CoV-2 pandemic and one of the countries with stringent mother-neonate isolation measure implemented. Now increasing evidence suggests that coronavirus disease 2019 (COVID-19) should not be taken as an indication for formula feeding or isolation of the infant from the mother. Methods We conducted a retrospective cohort study in 44 hospitals from 14 provinces in China to investigate the management of neonates whose mothers have confirmed or suspected COVID-19. In addition, 65 members of Chinese Neonatologist Association (CNA) were invited to give their comments and suggestions on the clinical management guidelines for high-risk neonates. Results There were 121 neonates born to 118 mothers suspected with COVID-19 including 42 mothers with SARS-CoV-2 positive results and 76 mothers with SARS-CoV-2 negative results. All neonates were born by caesarean section, isolated from their mothers immediately after birth and were formula-fed. Five neonates were positive for SARSCoV-2 at initial testing between 36 and 46 h after birth. Regarding the confusion on the clinical management guidelines, 58.78% of the newborns were put into isolation, 32.22% were subject to PCR tests, and 5.16% and 2.75% received breastfeeding and vaccination, respectively. Conclusion The clinical symptoms of neonates born to mothers with confirmed SARS-CoV-2 were mild, though five neonates might have been infected in utero or during delivery. Given the favorable outcomes of neonates born to COVID-confirmed mothers, full isolation may not be warranted. Rather, separation of the mother and her newborn should be assessed on a case-by-case basis, considering local facilities and risk factors for adverse outcomes, such as prematurity and fetal distress. -
Key words:
- coronavirus disease 2019 /
- neonates /
- pregnant women /
- infection control
-
Table 1. Clinical characteristics of mothers with confirmed or suspected COVID-19
Characteristics Mothers positive for SARS-CoV-2
(N = 42)Mothers negative for SARS-CoV-2
(N = 76)P value Age (years) 0.448 20-24 1(2.4%) 5(6.6%) 25-29 16(38.1%) 30(39.5%) 30-34 20(47.6%) 27(35.5%) ≥35 5 (11.9%) 14(18.4%) Caesarean section, N(%) 37(88.1%) 48(63.2%) 0.004 Complications during pregnancy Diabetes, N (%) 16(38.1%) 16(21.0%) 0.046 Hypertension, N(%) 10(23.8%) 7(9.2%) 0.031 History of exposure to COVID-19 41(97.6%) 67(88.2%) 0.155 Signs and symptoms Fever, N (%) 28(66.7%) 49(64.5%) 0.811 Cough or sorethroat, N (%) 21(50.0%) 18(23.7%) 0.004 Neutrophil count < 9.5×109/L 37 (94.9%)* 40 (53.3%)* < 0.001 Lymphocyte count (< 1×109/L) 4 (10.3%) 8 (10.7%) 1.000 Abnormal Chest imaging, N (%) 39 (95.1%)δ 45 (72.6%)δ 0.009 *39 of 42 mothers with confirmed COVID-19 and 75/76 mothers with negative test results had routine blood results. δ41 of 42 mothers with confirmed COVID-19 and 62/76 mothers with negative test results had chest imaging. Table 2. Clinical characteristics of neonates born to mothers with confirmed or suspected COVID-19
Neonates born to mothers with
confirmed COVID-19
(N = 44)*Neonates born to mothers
without confirmed COVID-19
(N = 77)#t/χ2 P value Gestational age at delivery, N (%) 3.422 0.181 ≤33 week 5(11.4) 8(10.4) 34-36 week 10(22.7) 8(10.4) 37-41 week 29(65.9) 60(77.92) ≥42 week 0 0 Premature, N (%) 15(34.1) 16(20.8) 2.604 0.107 Birth weight, N (%) 2.033 0.566 ≤1000 g 0 0 > 1 000-1 500 g 0 2(2.6) > 1 500-2 500 g 11 (25.0) 14(18.2) > 2 500-4 000 g 32(72.7) 60(77.9) > 4 000 g 1(2.3) 1(1.3) Female sex, N (%) 18(40.9) 35(45.5) 0.235 0.628 Apgar score, N (%) ≤7 at 1 min after birth 5(11.4) 4(5.2) 0.781 0.377 ≤7 at 5 min after birth 3(6.8) 1(1.4) 1.221 0.269 Signs and symptoms Fever (≥ 37.3℃), N (%) 15(34.1) 10(13.0) 7.608 0.006 Dyspnea, N(%) 14(31.8) 25(32.5) 0.005 0.941 No symptoms, N (%) 20(45.5) 49(63.6) 3.777 0.052 Chest imaging Abnormal, N (%) 24(60.0)$ 25(46.3)Φ 1.729 0.189 Feeding mode, N (%) Exclusively Breastfeeding 0 2(2.6) 0.113 0.736 Formula feeding 40(90.9) 62(80.5) 2.283 0.131 Mixed feeding 4(9.1) 9(11.70) 0.019 0.890 Nil by mouth 0 4(5.2) 1.018 0.313 SARS-CoV-2 PCR Isolation unit, N (%) 44(100) 50(64.9) Performed, N (%) Not isolated 1(2.3) 0 Primary care 14(31.8) 58(75.3) 24.037 0 Special care 29(65.9) 19(25.7) Intensive care 0 0 *Includes 2 pairs of twins. #Includes 1 pair of twins. $40 of 44 cases in neonates born to mothers with prenatally confirmed COVID-19 had chest imaging. Φ54 of 77 cases in neonates born to mothers with prenatally suspected/postnatally PCR-negative had chest imaging. Table 3. Clinical characteristics of neonates with positive PCR SARS-CoV-2 testing and select characteristics of their mothers
Neonate 1 Neonate 2 Neonate 3 Neonate 4 Neonate 5 Neonate 6 Mothers Age (years) 32 32 33 30 32 27 Diabetes No No No No No No Hypertension No No No No No No Fever No No Yes No Yes Yes Cough or sore throat No No Yes No No Yes Chest radiography Normal Normal Abnormal Abnormal Abnormal Abnormal Leukocyte count (×109/L) 6.3 6.3 3.6 8.8 6.9 7.9 PCR-positive prior to delivery Yes Yes Yes Yes Yes No Neonates Gestational age 31w+2 31w+2 40w+0 40w+6 39w+1 38w+5 Birthweight (g) 1580 1580 3250 3360 3500 3300 Caesarean section Yes Yes Yes Yes Yes Yes Apgar score (1 min, 5 min) 3, 4 8, 9 8, 9 9, 10 9, 10 9, 10 Fever No No Yes Yes Yes Yes Tachypnea Yes Yes No Yes No No Chest radiography Abnormal Abnormal Abnormal Abnormal Abnormal Abnormal Leukocyte count (×109/L) 4.8 4.3 7.4 17.9 12.2 7.3 SARS-CoV-2 PCR test result* 1st testing 36 h 36 h 40 h 46 h 38 h 5 days Nasal pharyngeal
swab (+)
Anal swab (+)Nasal pharyngeal
swab (+)
Anal swab (+)Nasal pharyngeal
swab (+)
Anal swab (+)Nasal pharyngeal
swab (+)
Anal swab (+)Nasal pharyngeal
swab (+)
Anal swab (+)Nasal pharyngeal
swab (+)
Anal swab (+)2nd testing 3 days 4 days 4 days 3 days 4 days N/A Nasal pharyngeal
swab (-)
Anal swab N/ANasal pharyngeal
swab (+)
Anal swab N/ANasal pharyngeal
swab (+)
Anal swab (+)Nasal pharyngeal
swab (+)
Anal swab N/ANasal pharyngeal
swab (-), Anal
swab N/AIsolated immediately after birth Yes Yes Yes Yes Yes No Breastfeeding or received expressed breast milk No No No No No No Outcome Discharged Discharged Discharged Discharged Discharged Discharged *Hours or days after birth -
[1] World Health Organization. Coronavirus disease (COVID-19) Situation dashboard. Geneva: World Health Organization, 2021. [2] National Health Commission of the People's Republic of China. The latest situation of novel coronavirus pneumonia as of 24: 00 on 17 September 2021. Available at: http://www.nhc.gov.cn/wjw/index.shtml. Accessed on Sep 17, 2021. (Chinese). [3] Yang J, Feng Z C. On behalf of the Chinese Neonatologist Association. Proposed management on 2019 novel coronavirus infection in neonates. Chin J Perinat Med, 2020; 23: 80-82. (Chinese). [4] Wang J H, Qi H B, Bao L, et al. A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units. Lancet Child Adolesc Health, 2020; 4(4): 258-259. doi: 10.1016/S2352-4642(20)30040-7 [5] Li F, Feng Z C, Shi Y. Proposal for prevention and control of the 2019 novel coronavirus disease in newborn infants. Arch Dis Child Fetal Neonatal Ed, 2020; 105(6): fetalneonatal-2020-318996. [6] Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med, 2020; 382(25): e100. doi: 10.1056/NEJMc2009226 [7] Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet, 2020; 395(10226): 809-815. doi: 10.1016/S0140-6736(20)30360-3 [8] Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr, 2020; 174(7): 722-725. doi: 10.1001/jamapediatrics.2020.0878 [9] Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr, 2020; 9(1): 51-60. doi: 10.21037/tp.2020.02.06 [10] Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis, 2020; 20(5): 559-564. doi: 10.1016/S1473-3099(20)30176-6 [11] Walker K F, O'Donoghue K, Grace N, Dorling J, Comeau J L, Li W, et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG, 2020;127(11): 1324-1336. doi: 10.1111/1471-0528.16362 [12] Salvatore C M, Han J Y, Acker K P, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health, 2020; 4(10): 721-727. doi: 10.1016/S2352-4642(20)30235-2 [13] National Health Commission of the People's Republic of China. New coronavirus pneumonia prevention and control program (6th edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Accessed on Oct 5, 2020. [14] National Health Commission of the People's Republic of China. Guideline for laboratory testing of new coronary virus pneumonia. Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/a5d6f7b8c48c451c87dba14889b30147/files/3514cb996ae24e2faf65953b4ecd0df4.pdf. Accessed on Oct 5, 2020. (Chinese). [15] Lokken E M, Walker C L, Delaney S, et al. Clinical characteristics of 46 pregnant women with a SARS-CoV-2 infection in Washington State. Am J Obstet Gynecol, 2020; 223(6): 911. e1-911. e14. [16] Schmid MB, Fontijn J, Ochsenbein-Kölble N, et al. COVID-19 in pregnant women. Lancet Infect Dis, 2020; 20(6): 652-653. doi: 10.1016/S1473-3099(20)30157-2 [17] Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM, 2020; 2(2): 100118. doi: 10.1016/j.ajogmf.2020.100118 [18] Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women hospitalised with confirmed SARS-CoV-2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS). BMJ, 2020; 369: m2107. [19] Landelle C, Pagani L, Harbarth S. Is patient isolation the single most important measure to prevent the spread of multidrug-resistant pathogens? Virulence, 2013; 4(2): 163-171. doi: 10.4161/viru.22641 [20] Sprague E, Reynolds S, Brindley P. Patient isolation precautions: are they worth it? Canadian Respiratory Journal, 2016; 2016: 5352625. [21] World Health Organization. Q & A: Breastfeeding and COVID-19. Available at: https://www.who.int/emergencies/diseases/novelcoronavirus-2019/question-and-answers-hub/q-a-detail/q-a-on-covid-19-and-breastfeeding. Accessed on May 7, 2020. [22] Chambers C D, Krogstad P, Bertrand K, et al. Evaluation of SARSCoV-2 in breastmilk from 18 infected women. medrXiv 2020; published online June 16. DOI: 10.1101/2020.06.12.20127944(preprint). [23] AAP issues guidance on infants born to mothers with suspected or confirmed COVID-19. Available at: https://www.aappublications.org/news/2020/04/02/infantcovidguidance040220. Accessed on May 7, 2020. [24] Favre G, Pomar L, Qi X, et al. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect Dis, 2020; 20(6): 652-653. doi: 10.1016/S1473-3099(20)30157-2 [25] Salvatore C M, Han J Y, Acker K P, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health, 2020, 4(10): 721-727. doi: 10.1016/S2352-4642(20)30235-2 -


 投稿系统
投稿系统


 下载:
下载: