Dissecting pathophysiology of a human dominantly inherited disease, familial amyloidotic polyneuropathy, by using genetically engineered mice
doi: 10.2478/fzm-2022-0009
-
Abstract: Familial amyloidotic polyneuropathy (FAP) is a type of systemic amyloidosis characterized by peripheral and autonomic neuropathy. Although FAP is a typical autosomal dominant disorder caused by a point mutation in the TTR gene, the average age at onset varies significantly among different countries. This discrepancy clearly suggests that a combination of intrinsic factors as well as extrinsic (environmental) factors shapes the development of FAP. However, these factors are difficult to analyze in humans, because detailed pathologic tissue analysis is only possible at autopsy. Thus, mouse models have been produced and used to disentangle these factors. This review covers the mouse models produced thus far and how these models are applied to analyze intrinsic and extrinsic factors involved in disease development and to test drug efficacy.
-
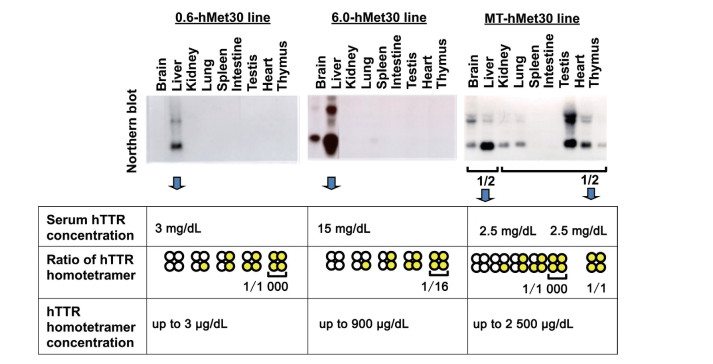
Figure 1. Relationship between the concentration of hTTR homotetramer and the amount of amyloid deposition
Concentrations of hTTR homotetramer were estimated from the amounts of TTR mRNA and the serum hTTR concentrations. Amounts of amyloid deposition were correlated with the concentration of the hTTR homotetramer.
Figure 2. Effect of intestinal flora on amyloid deposition
The 6.0-hM30 mice were kept under SPF or CV conditions. Amyloid deposition was observed only in mice maintained under CV conditions. Under SPF conditions, the intestinal flora remained fairly constant, and the major flora was group Ⅰ symbiotes, such as gram-positive anaerobic cocci. In contrast, under CV conditions, the group Ⅲ weak pathogens such as yeast, staphylococci, and Pseudomonas, increased, while that of group Ⅰ symbiotes decreased. GPAC, gram positive anaerobic cocci; GPAR, gram positive anaerobic rods.
Figure 3. Role of Cys10 in amyloid deposition
Three recombinant cDNAs encoding normal hTTRC10:V30, hTTRC10:M30, and hTTRSer10:Met30 were introduced into the cDNA cloning site of the plasmid pTG.27; the resultant plasmids were designated 7.2-hC10:V30c, 7.2-hC10:M30c, and 7.2-hS10:M30c, respectively. Amyloid deposition was observed only in 7.2-hC10:M30c mice.
Table 1. Serum TTR concentrations and amounts of amyloid deposition
Author Mouse Frequency of amyloid deposition Construct Ttr Serum conc. up tp 5 months 6 – 11 months 12 – 17 months 18 – 24 months Kohno 1997[31] 6.0-hTTRV30M +/+ 30 – 65 μg/mL not done 1/6 2/6 4/6 6.0-hTTRV30M −/− 22 – 79 μg/mL not done 3/6 2/6 3/6 Takaoka 2004[19] 7.2-hTTRV30 +/+ 38 – 53 μg/mL not done 0/1 0/8 0/27 7.2-hTTRC10:V30M +/+ 48 – 57 μg/mL not done 0/4 0/19 14/27 7.2-hTTRC10S: V30M +/+ 4 – 78 μg/mL not done 0/4 0/11 0/40 Inoue 2008[30] 0.6-hTTRV30M +/+ 15 – 30 μg/mL not done 0/2 4/8 4/6 6.0-hTTRV30M +/+ 137 – 145 μg/mL not done 0/12 7/11 6/6 MT-hTTRV30M +/+ 10 – 48 μg/mL not done 1/8 10/13 7/7 Teng 2001[21] 19.2kb hTTRV30 +/+ 1000 – 3500 μg/mL not done not done 0/15 13/83 19.2kb hTTRL55P +/+ 10 – 30 μg/mL not done not done 0 0 Sousa 2002[29] MT-hTTRL55P −/− 50 – 200 μg/mL 0/21 0/12 0/11 0/11 6.0-hTTRV30M +/+ not done 0/24 0/25 0/25 1/25 Ttr−/− : 6.0-hTTRV30M −/− not done 1/1 0/2 13/13 16/16 Li 2018[80] Ttr+/+ : 6.0-hTTRV30M +/+ 142 – 149 μg/mL not done 8/10 6/7 12/12 Ttr−/− : 6.0-hTTRV30M −/− 131 – 143 μg/mL not done 5/10 5/7 12/12 TtrV30/V30: Rbp4RBP4 −/− 6.0 – 6.6 μg/mL not done 10/10 7/8 12/12 TtrV30/M30: Rbp4RBP4 −/− 5.5 – 5.9 μg/mL not done 0/10 6/6 8/8 The amounts of amyloid deposition varied greatly depending on housing conditions, type of mutation, etc. For example, Sousa et al. reported that amyloid deposition was observed only one out of 25 mice, while we reported that amyloid deposition was observed most of mice even though both groups used the same 6.0-hTTRV30M. Table 2. Amyloid deposition in various Tg Lines
Tg Lines Tissue 6 months 12 months 18 months 24 months 0.6-hMet30 heart − − − ~ + − small intestine − − + ~ + + − ~ + kidney − − − − skin − − − ~ + − sciatic nerve − − − − 6.0-hMet30 heart − − ~ + − + ~ + + + small intestine − − ~ + + − ~ + + + kidney − − ~ ± − + + + skin − − ~ + − − ~ + sciatic nerve − − − − MT-hMet30 heart − − ~ + + ~ + + + + + small intestine − ~ + − ~ + + + + + + + + kidney − − ~ + + + ~ + + + + + + skin − + + + + sciatic nerve − − − − Amyloid deposition in 6.0-h Met30 was earlier than that in 0.6-h Met30. This was expected from the data that serum TTR level was higher in 6.0-h Met30 than in 0.6-h Met30 line. However, amyloid deposition in MT-h Met30 was earlier and larger than those in other lines. -
[1] Andrade C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain, 1952; 75(3): 408-427. doi: 10.1093/brain/75.3.408 [2] Dickson P W, Howlett G J, Schreiber G. Rat transthyretin (prealbumin). Molecular cloning, nucleotide sequence, and gene expression in liver and brain. J Biol Chem, 1985; 260(13): 8214-8219. doi: 10.1016/S0021-9258(17)39583-2 [3] Kato M, Soprano D R, Makover A, et al. Localization of immunoreactive transthyretin (prealbumin) and of transthyretin mRNA in fetal and adult rat brain. Differentiation, 1986; 31(3): 228-235. doi: 10.1111/j.1432-0436.1986.tb00402.x [4] Soprano D R, Herbert J, Soprano K J, et al. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem, 1985; 260(21): 11793-11798. doi: 10.1016/S0021-9258(17)39100-7 [5] Schreiber G. The evolution of transthyretin synthesis in the choroid plexus. Clin Chem Lab Med, 2002; 40(12): 1200-1210. [6] Vranckx R, Savu L, Maya M, et al. Characterization of a major development-regulated serum thyroxine-binding globulin in the euthyroid mouse. Biochem J, 1990; 271(2): 373-379. doi: 10.1042/bj2710373 [7] Blake C C, Swan I D, Rerat C, et al. An X-ray study of the subunit structure of prealbumin. J Mol Biol, 1971; 61(1): 217-224. doi: 10.1016/0022-2836(71)90218-X [8] Colon W, Kelly J W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry, 1992; 31(36): 8654- 8660. doi: 10.1021/bi00151a036 [9] Hammarstrom P, Jiang X, Hurshman A R, et al. Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A, 2002; 99(Suppl 4): 16427-16432. [10] Lai Z, Colon W, Kelly J W. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can selfassemble into amyloid. Biochemistry, 1996; 35(20): 6470-6482. doi: 10.1021/bi952501g [11] Hund E, Linke R P, Willig F, et al. Transthyretin-associated neuropathic amyloidosis. Pathogenesis and treatment. Neurology, 2001; 56(4): 431-435. http://www.ncbi.nlm.nih.gov/pubmed/11261421 [12] Ikeda S, Hanyu N, Hongo M, et al. Hereditary generalized amyloidosis with polyneuropathy. Clinicopathological study of 65 Japanese patients. Brain, 1987; 110 (Pt 2): 315-337. [13] Plante-Bordeneuve V, Lalu T, Misrahi M, et al. Genotypic-phenotypic variations in a series of 65 patients with familial amyloid polyneuropathy. Neurology, 1998; 51(3): 708-714. doi: 10.1212/WNL.51.3.708 [14] Reilly M M, Adams D, Booth D R, et al. Transthyretin gene analysis in European patients with suspected familial amyloid polyneuropathy. Brain, 1995; 118 (Pt 4): 849-856. [15] Sousa A, Andersson R, Drugge U, et al. Familial amyloidotic polyneuropathy in Sweden: geographical distribution, age of onset, and prevalence. Hum Hered, 1993; 43(5): 288-294. doi: 10.1159/000154146 [16] Yamamura K, Wakasugi S, Maeda S, et al. Tissue-specific and developmental expression of human transthyretin gene in transgenic mice. Dev Genet, 1987; 8(4): 195-205. doi: 10.1002/dvg.1020080404 [17] Nagata Y, Tashiro F, Yi S, et al. A 6-kb upstream region of the human transthyretin gene can direct developmental, tissue-specific, and quantitatively normal expression in transgenic mouse. J Biochem, 1995; 117(1): 169-175. doi: 10.1093/oxfordjournals.jbchem.a124705 [18] Tagoe C E, Jacobson D R, Gallo G, et al. Mice transgenic for human TTR have the same frequency of renal TTR deposition whether maintained in conventional or specific pathogen free environments. Amyloid, 2003; 10(4): 262-266. doi: 10.3109/13506120309041744 [19] Takaoka Y, Ohta M, Miyakawa K, et al. Cysteine 10 is a key residue in amyloidogenesis of human transthyretin Val30Met. Am J Pathol, 2004; 164(1): 337-345. doi: 10.1016/S0002-9440(10)63123-9 [20] Takaoka Y, Tashiro F, Yi S, et al. Comparison of amyloid deposition in two lines of transgenic mouse that model familial amyloidotic polyneuropathy, type Ⅰ. Transgenic Res, 1997; 6(4): 261-269. doi: 10.1023/A:1018454527309 [21] Teng M H, Yin J Y, Vidal R, et al. Amyloid and nonfibrillar deposits in mice transgenic for wild-type human transthyretin: a possible model for senile systemic amyloidosis. Lab Invest, 2001; 81(3): 385-396. doi: 10.1038/labinvest.3780246 [22] Watts R P, Umemichi T, Zeldenrust S R, et al. Development of lines of transgenic mice expressing the human transthyretin Ser84 variant. Neuromuscul Disord, 1996; 6(Sul 1): S31. [23] Yi S, Takahashi K, Naito M, et al. Systemic amyloidosis in transgenic mice carrying the human mutant transthyretin (Met30) gene. Pathologic similarity to human familial amyloidotic polyneuropathy, type Ⅰ. Am J Pathol, 1991; 138(2): 403-412. [24] Costa P P, Figueira A S, Bravo F R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A, 1978; 75(9): 4499-4503. doi: 10.1073/pnas.75.9.4499 [25] Dwulet F E, Benson M D. Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc Natl Acad Sci U S A, 1984; 81(3): 694-698. doi: 10.1073/pnas.81.3.694 [26] Saraiva M J, Birken S, Costa P P, et al. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest, 1984; 74(1): 104-119. doi: 10.1172/JCI111390 [27] Tawara S, Nakazato M, Kangawa K, et al. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun, 1983; 116(3): 880-888. doi: 10.1016/S0006-291X(83)80224-1 [28] Harats N, Worth R O, Benson M D. Evidence against early amyloid deposition in heredutary amyloidosis, vol. 3. Portugal: Arquivos de Medicina, 1990. [29] Sousa M M, Fernandes R, Palha J A, et al. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol 2002; 161(5): 1935-1948. doi: 10.1016/S0002-9440(10)64469-0 [30] Inoue S, Ohta M, Li Z, et al. Specific pathogen free conditions prevent transthyretin amyloidosis in mouse models. Transgenic Res, 2008; 17(5): 817-826. doi: 10.1007/s11248-008-9180-9 [31] Kohno K, Palha J A, Miyakawa K, et al. Analysis of amyloid deposition in a transgenic mouse model of homozygous familial amyloidotic polyneuropathy. Am J Pathol, 1997; 150(4): 1497-1508. [32] Li X, Lyu Y, Shen J, et al. Amyloid deposition in a mouse model humanized at the transthyretin and retinol-binding protein 4 loci. Lab Invest, 2018; 98(4): 512-524. doi: 10.1038/s41374-017-0019-y [33] Bergstrom J, Gustavsson A, Hellman U, et al. Amyloid deposits in transthyretin-derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. J Pathol, 2005; 206(2): 224-232. doi: 10.1002/path.1759 [34] Ihse E, Rapezzi C, Merlini G, et al. Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid, 2013; 20(3): 142-150. doi: 10.3109/13506129.2013.797890 [35] Ihse E, Suhr O B, Hellman U, et al. Variation in amount of wild-type transthyretin in different fibril and tissue types in ATTR amyloidosis. J Mol Med (Berl), 2011; 89(2): 171-180. doi: 10.1007/s00109-010-0695-1 [36] Ihse E, Ybo A, Suhr O, et al. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol, 2008; 216(2): 253-261. doi: 10.1002/path.2411 [37] Mangione P P, Porcari R, Gillmore J D, et al. Proteolytic cleavage of Ser52Pro variant transthyretin triggers its amyloid fibrillogenesis. Proc Natl Acad Sci U S A, 2014; 111(4): 1539-1544. doi: 10.1073/pnas.1317488111 [38] Marcoux J, Mangione P P, Porcari R, et al. A novel mechanoenzymatic cleavage mechanism underlies transthyretin amyloidogenesis. EMBO Mol Med, 2015; 7(10): 1337-1349. doi: 10.15252/emmm.201505357 [39] Sant'Anna R, Braga C, Varejao N, et al. The importance of a gatekeeper residue on the aggregation of transthyretin: implications for transthyretin-related amyloidoses. J Biol Chem, 2014; 289(41): 28324- 28337. doi: 10.1074/jbc.M114.563981 [40] Mangione P P, Verona G, Corazza A, et al. Plasminogen activation triggers transthyretin amyloidogenesis in vitro. J Biol Chem, 2018; 293(37): 14192-14199. doi: 10.1074/jbc.RA118.003990 [41] Slamova I, Adib R, Ellmerich S, et al. Plasmin activity promotes amyloid deposition in a transgenic model of human transthyretin amyloidosis. Nat Commun, 2021; 12(1): 7112. doi: 10.1038/s41467-021-27416-z [42] Bohrmann B, Tjernberg L, Kuner P, et al. Endogenous proteins controlling amyloid beta-peptide polymerization. Possible implications for beta-amyloid formation in the central nervous system and in peripheral tissues. J Biol Chem, 1999; 274(23): 15990-15995. doi: 10.1074/jbc.274.23.15990 [43] Gollin P A, Kalaria R N, Eikelenboom P, et al. Alpha 1-antitrypsin and alpha 1-antichymotrypsin are in the lesions of Alzheimer's disease. Neuroreport, 1992; 3(2): 201-203. doi: 10.1097/00001756-199202000-00020 [44] Torricelli C, Capurro E, Santucci A, et al. Multiple plasma proteins control atrial natriuretic peptide (ANP) aggregation. J Mol Endocrinol, 2004; 33(2): 335-341. doi: 10.1677/jme.1.01530 [45] Sharp H L. The current status of alpha-1-antityrpsin, a protease inhibitor, in gastrointestinal disease. Gastroenterology, 1976; 70(4): 611- 621. doi: 10.1016/S0016-5085(76)80506-9 [46] Niemietz C, Fleischhauer L, Sandfort V, et al. Hepatocyte-like cells reveal novel role of SERPINA1 in transthyretin amyloidosis. J Cell Sci, 2018; 131(23): jcs219824. [47] Niemietz C, Bezerra F, Almeida M R, et al. SERPINA1 modulates expression of amyloidogenic transthyretin. Exp Cell Res, 2020; 395(2): 112217. doi: 10.1016/j.yexcr.2020.112217 [48] Bezerra F, Niemietz C, Schmidt H H J, et al. In vitro and in vivo effects of serpinA1 on the modulation of transthyretin proteolysis. Int J Mol Sci, 2021; 22(17): 9488. doi: 10.3390/ijms22179488 [49] Santos S D, Fernandes R, Saraiva M J. The heat shock response modulates transthyretin deposition in the peripheral and autonomic nervous systems. Neurobiol Aging, 2010; 31(2): 280-289. doi: 10.1016/j.neurobiolaging.2008.04.001 [50] Tagoe C E, Reixach N, Friske L, et al. In vivo stabilization of mutant human transthyretin in transgenic mice. Amyloid, 2007; 14(3): 227-236. doi: 10.1080/13506120701464396 [51] Reixach N, Foss T R, Santelli E, et al. Human-murine transthyretin heterotetramers are kinetically stable and non-amyloidogenic. A lesson in the generation of transgenic models of diseases involving oligomeric proteins. J Biol Chem, 2008; 283(4): 2098-2107. doi: 10.1074/jbc.M708028200 [52] Soprano D R, Blaner W S. Plasma retino-binding protein. New York: Raven Press, 1994. [53] Benson M D, Kincaid J C. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve, 2007; 36(4): 411-423. doi: 10.1002/mus.20821 [54] Dyck P J, Lambert E H. Dissociated sensation in amylidosis. Compound action potential, quantitative histologic and teased-fiber, and electron microscopic studies of sural nerve biopsies. Arch Neurol, 1969; 20(5): 490-507. doi: 10.1001/archneur.1969.00480110054005 [55] Sousa M M, Du Yan S, Fernandes R, et al. Familial amyloid polyneuropathy: receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J Neurosci, 2001; 21(19): 7576-7586. doi: 10.1523/JNEUROSCI.21-19-07576.2001 [56] Sousa M M, Cardoso I, Fernandes R, et al. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am J Pathol, 2001; 159(6): 1993-2000. doi: 10.1016/S0002-9440(10)63050-7 [57] Murakami T, Sango K, Watabe K, et al. Schwann cells contribute to neurodegeneration in transthyretin amyloidosis. J Neurochem, 2015; 134(1): 66-74. doi: 10.1111/jnc.13068 [58] Chao C C, Huang C M, Chiang H H, et al. Sudomotor innervation in transthyretin amyloid neuropathy: Pathology and functional correlates. Ann Neurol, 2015; 78(2): 272-283. doi: 10.1002/ana.24438 [59] Yang N C, Lee M J, Chao C C, et al. Clinical presentations and skin denervation in amyloid neuropathy due to transthyretin Ala97Ser. Neurology, 2010; 75(6): 532-538. doi: 10.1212/WNL.0b013e3181ec7fda [60] Kan H W, Chiang H, Lin W M, et al. Sensory nerve degeneration in a mouse model mimicking early manifestations of familial amyloid polyneuropathy due to transthyretin Ala97Ser. Neuropathol Appl Neurobiol, 2018; 44(7): 673-686. doi: 10.1111/nan.12477 [61] Hafer-Macko C E, Dyck P J, Koski C L. Complement activation in acquired and hereditary amyloid neuropathy. J Peripher Nerv Syst, 2000; 5(3): 131-139. doi: 10.1046/j.1529-8027.2000.00018.x [62] Dardiotis E, Koutsou P, Zamba-Papanicolaou E, et al. Complement C1Q polymorphisms modulate onset in familial amyloidotic polyneuropathy TTR Val30Met. J Neurol Sci, 2009; 284(1-2): 158-162. doi: 10.1016/j.jns.2009.05.018 [63] Panayiotou E, Fella E, Papacharalambous R, et al. C1q ablation exacerbates amyloid deposition: A study in a transgenic mouse model of ATTRV30M amyloid neuropathy. PLoS One, 2017; 12(4): e0175767. doi: 10.1371/journal.pone.0175767 [64] Fonseca M I, Chu S H, Berci A M, et al. Contribution of complement activation pathways to neuropathology differs among mouse models of Alzheimer's disease. J Neuroinflammation, 2011; 8(1): 4. doi: 10.1186/1742-2094-8-4 [65] Mathern D R, Heeger P S. Molecules great and small: the complement system. Clin J Am Soc Nephrol, 2015; 10(9): 1636-1650. doi: 10.2215/CJN.06230614 [66] Fonseca M I, Ager R R, Chu S H, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J Immunol, 2009; 183(2): 1375- 1383. doi: 10.4049/jimmunol.0901005 [67] Fella E, Sokratous K, Papacharalambous R, et al. Pharmacological stimulation of phagocytosis enhances amyloid plaque clearance; evidence from a transgenic mouse model of ATTR neuropathy. Front Mol Neurosci, 2017; 10: 138. doi: 10.3389/fnmol.2017.00138 [68] Michalon A, Hagenbuch A, Huy C, et al. A human antibody selective for transthyretin amyloid removes cardiac amyloid through phagocytic immune cells. Nat Commun, 2021; 12(1): 3142. doi: 10.1038/s41467-021-23274-x [69] Pepys M B, Dash A C. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet, 1977; 1(8020): 1029-1031. http://www.onacademic.com/detail/journal_1000036178038010_b14f.html [70] Pepys M B, Dyck R F, de Beer F C, et al. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol, 1979; 38(2): 284-293. [71] Sorboni S G, Moghaddam H S, Jafarzadeh-Esfehani R, et al. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev, 2022; 35(1): e0033820. doi: 10.1128/CMR.00338-20 [72] Noguchi H, Ohta M, Wakasugi S, et al. Effect of the intestinal flora on amyloid deposition in a transgenic mouse model of familial amyloidotic polyneuropathy. Exp Anim, 2002; 51(4): 309-316. doi: 10.1538/expanim.51.309 [73] Murakami T, Yi S, Maeda S, et al. Effect of serum amyloid P component level on transthyretin-derived amyloid deposition in a transgenic mouse model of familial amyloidotic polyneuropathy. Am J Pathol, 1992; 141(2): 451-456. [74] Terry C J, Damas A M, Oliveira P, et al. Structure of Met30 variant of transthyretin and its amyloidogenic implications. The EMBO Journal, 1993; 12(2): 735-741. doi: 10.1002/j.1460-2075.1993.tb05707.x [75] Ohta M, Sugano A, Hatano N, et al. Co-precipitation molecules hemopexin and transferrin may be key molecules for fibrillogenesis in TTR V30M amyloidogenesis. Transgenic Res, 2018; 27(1): 15-23. doi: 10.1007/s11248-017-0054-x [76] Zhao G, Li Z, Araki K, et al. Inconsistency between hepatic expression and serum concentration of transthyretin in mice humanized at the transthyretin locus. Genes Cells, 2008; 13(12): 1257-1268. doi: 10.1111/j.1365-2443.2008.01242.x [77] Liu L, Suzuki T, Shen J, et al. Rescue of retinal morphology and function in a humanized mouse at the mouse retinol-binding protein locus. Lab Invest, 2017; 97(4): 395-408. doi: 10.1038/labinvest.2016.156 [78] Zanotti G, Cendron L, Folli C, et al. Structural evidence for native state stabilization of a conformationally labile amyloidogenic transthyretin variant by fibrillogenesis inhibitors. FEBS Lett, 2013; 587(15): 2325-2331. doi: 10.1016/j.febslet.2013.06.016 [79] Mu Y, Jin S, Shen J, et al. CHF5074 (CSP-1103) stabilizes human transthyretin in mice humanized at the transthyretin and retinol-binding protein loci. FEBS Lett, 2015; 589(7): 849-856. doi: 10.1016/j.febslet.2015.02.020 [80] Li Z, Kanazashi H, Tokashiki Y, et al. TTR exon-humanized mouse optimal for verifying new therapies for FAP. Biochem Biophys Res Commun, 2022; 599: 69-74. doi: 10.1016/j.bbrc.2022.02.035 -


 投稿系统
投稿系统


 下载:
下载: