Apelin aggravates the migration and invasion of nonsmall cell lung cancer cells via YAP1
doi: 10.2478/fzm-2022-0007
-
Abstract:
Background Apelin, an endogenous ligand of G-protein coupled receptor (GPCR), is a secreted peptide involved in the development of various tumors. However, the relationship between apelin and nonsmall cell lung cancer (NSCLC) is not quite clear. This study was designed to investigate the effect and mechanism of apelin on cell proliferation, migration and invasion of NSCLC cells. Methods Twelve NSCLC specimens were collected for hematoxylin-eosin (HE) staining and immunohistochemistry analyses. Cell proliferation was examined by 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and cell migration and invasion were assessed using wound-healing and transwell assays. The subcellular location of yes associated protein 1 (YAP1) in A549 cells was determined by immunofluorescence. The mRNA and protein levels in NSCLC tissues and cell lines were measured by qRT-PCR and western blot, respectively. Results Apelin was upregulated in tumor tissues compared with the adjacent tissues. Apelin promoted proliferation, migration, and invasion of A549 and H460 cells, which was reversed by competitive apelin receptor (APJ) antagonist ML221. Additionally, apelin upregulated YAP1 expression, whereas silence of YAP1 by small interfering RNA (siRNA) attenuated apelin-induced cell proliferation, migration and invasion and suppressed epithelial-mesenchymal transition progression. Conclusion Apelin promotes NSCLC cells proliferation, migration, and invasion by modulating YAP1 and might be a potential therapeutic target for NSCLC treatment. -
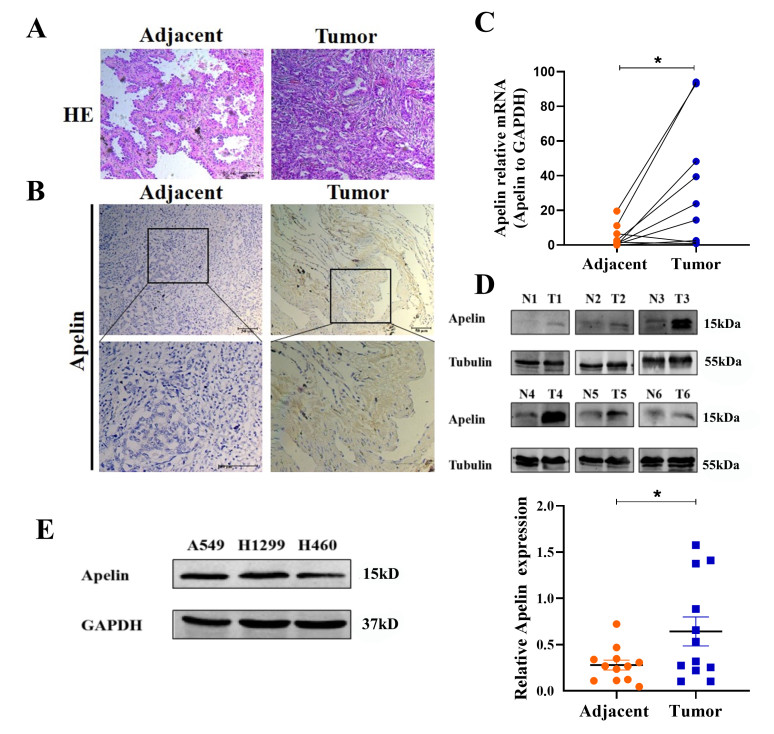
Figure 1. Exaggerated expression of apelin in NSCLC specimens
(A) HE staining of human NSCLC tissues and matched adjacent tissues. (B) Representative IHC imaging of apelin in NSCLC tissues. Scale bar, 50 μmol/L. (C) qRT-PCR analysis of apelin mRNA levels in human NSCLC tissues and paired adjacent tissues. N = 8, *P < 0.05. (D) Representative western-blot outcomes of apelin in 12 pairs of normal (N) and tumor (T) samples of NSCLC; Scatter diagram on the right panel represents relative apelin expression. N = 12, *P < 0.05. (E) Western-blot assay of apelin protein levels in three NSCLC cell lines was performed.
Figure 3. Apelin promotes A549 and H460 cells proliferation, migration and invasion via APJ
MTT analysis of cell viability in A549 (A) and H460 (B) cells with apelin and APJ antagonist ML221. Wound-healing assays for A549 cells (C) and H460 cells (D) with apelin and ML221 at 0, 24, 48 h after scratching. N = 6, *P < 0.05, **P < 0.01. Transwell assay detected the migration (E) and invasion (F) of A549 and H460 cells with apelin and ML221. Cell counts were for the corresponding assays of at least four random microscope fields. N = 3, **P < 0.01.
Figure 4. Apelin activates YAP1 signaling
(A and B) Immunofluorescence assays showed that the apelin upregulated YAP1 expression in nuclear in A549 and H460 cells. (C and D) Western-blot analysis showing the expression of YAP1 in the presence of apelin treatment in A549 and H460 cells. N = 6, *P < 0.05, **P < 0.01.
Figure 5. Apelin regulates cell migration and invasion through YAP1
Cell viability in A549 (A) and H460 (B) cells with YAP1 silencing. N = 6, *P < 0.05. Wound-healing assays of A549 (C) and H460 (D) cells with YAP1 silencing at 0, 24, 48 h after scratching. N = 6, *P < 0.05. The migration and invasion abilities of A549 (E) and H460 (F) cells after transfection with YAP1 siRNA. Cell counts were for the corresponding assays of at least four random microscope fields. N = 3, *P < 0.05.
Figure 6. Apelin regulates EMT program by modulating YAP1
(A and B) Western-blot analysis showed the expression of EMT-relevant proteins (FN-1, E-cadherin, Vimentin) after knockdown of YAP1 in the presence of apelin treatment. N = 6, *P < 0.05. (C and D) Effect of YAP1 on apelin induced EMT was determined by immunofluorescence in H460 and A549 cells.
-
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2020; 70(4): 313. [2] Zhang M, Yang W, Wang P, et al. CCL7 recruits cDC1 to promote antitumor immunity and facilitate checkpoint immunotherapy to non-small cell lung cancer. Nat Commun, 2020; 11(1): 6119. doi: 10.1038/s41467-020-19973-6 [3] Kwak E L, Bang Y J, Camidge D R, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med, 2010; 363(18): 1693-1703. doi: 10.1056/NEJMoa1006448 [4] Hamanaka N, Nakanishi Y, Mizuno T, et al. YES1 is a targetable oncogene in cancers harboring YES1 gene amplification. Cancer Res, 2019; 79(22): 5734-5745. doi: 10.1158/0008-5472.CAN-18-3376 [5] Sorli S C, Le Gonidec S, Knibiehler B, et al. Apelin is a potent activator of tumour neoangiogenesis. Oncogene, 2007; 26(55): 7692-7699. doi: 10.1038/sj.onc.1210573 [6] O'Carroll A M, Lolait S J, Harris L E, et al. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol, 2013; 219(1): R13-35. doi: 10.1530/JOE-13-0227 [7] Li Z, He Q, Wu C, et al. Apelin shorten QT interval by inhibiting Kir2.1/IK1 via a PI3K way in acute myocardial infarction. Biochem Bioph Res Co, 2019; 517(2): 272-277. doi: 10.1016/j.bbrc.2019.07.041 [8] Berta J, Kenessey I, Dobos J, et al. Apelin expression in human non-small cell lung cancer: role in angiogenesis and prognosis. J Thorac Oncol, 2010; 5(8): 1120-1129. doi: 10.1097/JTO.0b013e3181e2c1ff [9] Yang Y, Lv S Y, Ye W, et al. Apelin/APJ system and cancer. Clin Chim Acta, 2016; 457: 112-116. doi: 10.1016/j.cca.2016.04.001 [10] Yu F X, Zhao B, Guan K L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell, 2015; 163(4): 811-828. doi: 10.1016/j.cell.2015.10.044 [11] Zhang Z, Du J, Wang S, et al. OTUB2 promotes cancer metastasis via hippo-independent activation of YAP and TAZ. Mol Cell, 2019; 73(1): 7-21 e7. doi: 10.1016/j.molcel.2018.10.030 [12] Zanconato F, Piccolo S. Eradicating tumor drug resistance at its YAP-biomechanical roots. EMBO J, 2016; 35(5): 459-461. doi: 10.15252/embj.201593584 [13] Yu M, Chen Y, Li X, et al. YAP1 contributes to NSCLC invasion and migration by promoting Slug transcription via the transcription co-factor TEAD. Cell Death Dis, 2018; 9(5): 464. doi: 10.1038/s41419-018-0515-z [14] Liang H, Yu T, Han Y, et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer, 2018; 17(1): 119. doi: 10.1186/s12943-018-0870-5 [15] Wu M, Duan Q, Liu X, et al. MiR-155-5p promotes oral cancer progression by targeting chromatin remodeling gene ARID2. Biomed Pharmacother, 2020; 122: 109696. doi: 10.1016/j.biopha.2019.109696 [16] Li X, Yu T, Shan H, et al. lncRNA PFAL promotes lung fibrosis through CTGF by competitively binding miR-18a. FASEB J, 2018; 32(10): 5285-5297. doi: 10.1096/fj.201800055R [17] Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Bioph Res Co, 1998; 251(2): 471-476. doi: 10.1006/bbrc.1998.9489 [18] Cano Martinez L J, Coral Vazquez R M, Mendez J P, et al. Serum concentrations of apelin-17 isoform vary in accordance to blood pressure categories in individuals with obesity class 3. Clin Exp Hypertens, 2019; 41(2): 168-173. doi: 10.1080/10641963.2018.1462374 [19] Yan J, Wang A, Cao J, et al. Apelin/APJ system: an emerging therapeutic target for respiratory diseases. Cell Mol Life Sci, 2020; 77(15): 2919-2930. doi: 10.1007/s00018-020-03461-7 [20] Wu L, Chen L, Li L. Apelin/APJ system: a novel promising therapy target for pathological angiogenesis. Clin Chim Acta, 2017; 466: 78-84. doi: 10.1016/j.cca.2016.12.023 [21] Xie H, Yuan L Q, Luo X H, et al. Apelin suppresses apoptosis of human OBs. Apoptosis, 2007; 12(1): 247-254. doi: 10.1007/s10495-006-0489-7 [22] Yu X H, Tang Z B, Liu L J, et al. Apelin and its receptor APJ in cardiovascular diseases. Clin Chim Acta, 2014; 428: 1-8. doi: 10.1016/j.cca.2013.09.001 [23] Podgorska M, Pietraszek-Gremplewicz K, Nowak D. Apelin effects migration and invasion abilities of colon cancer cells. Cells, 2018; 7(8): 113. doi: 10.3390/cells7080113 [24] Lv D, Li L, Lu Q, et al. PAK1-cofilin phosphorylation mediates human lung adenocarcinoma cells migration induced by apelin-13. Clin Exp Pharmacol Physiol, 2016; 43(5): 569-579. doi: 10.1111/1440-1681.12563 [25] Masoumi J, Jafarzadeh A, Khorramdelazad H, et al. Role of Apelin/APJ axis in cancer development and progression. Adv Med Sci, 2020; 65(1): 202-213. doi: 10.1016/j.advms.2020.02.002 [26] Plouffe S W, Hong A W, Guan K L. Disease implications of the Hippo/YAP pathway. Trends Mol Med, 2015; 21(4): 212-222. doi: 10.1016/j.molmed.2015.01.003 [27] Cao J J, Zhao X M, Wang D L, et al. YAP is overexpressed in clear cell renal cell carcinoma and its knockdown reduces cell proliferation and induces cell cycle arrest and apoptosis. Oncol Rep, 2014; 32(4): 1594-1600. doi: 10.3892/or.2014.3349 [28] Malik S A, Khan M S, Dar M, et al. Molecular alterations and expression dynamics of LATS1 and LATS2 genes in non-small-cell lung carcinoma. Pathol Oncol Res, 2018; 24(2): 207-214. doi: 10.1007/s12253-017-0225-3 [29] Pan D. The hippo signaling pathway in development and cancer. Dev Cell, 2010; 19(4): 491-505. doi: 10.1016/j.devcel.2010.09.011 [30] Mao X Y, Li Q Q, Gao Y F, et al. Gap junction as an intercellular glue: Emerging roles in cancer EMT and metastasis. Cancer Lett, 2016; 381(1): 133-137. doi: 10.1016/j.canlet.2016.07.037 [31] Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell, 2007; 130(6): 1120-1133. doi: 10.1016/j.cell.2007.07.019 [32] Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell, 2011; 147(4): 759-772. [33] Gao J, He L, Zhou L, et al. Mechanical force regulation of YAP by F-actin and GPCR revealed by super-resolution imaging. Nanoscale, 2020; 12(4): 2703-2714. [34] Dorsam R T, Gutkind J S. G-protein-coupled receptors and cancer. Nat Rev Cancer, 2007; 7(2): 79-94. [35] Yu F X, Zhang Y, Park H W, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Gene Dev, 2013; 27(11): 1223-1232. [36] Yu F X, Mo J S, Guan K L. Upstream regulators of the Hippo pathway. Cell Cycle, 2012; 11(22): 4097-4098. [37] Luo J, Yu F X. GPCR-Hippo Signaling in Cancer. Cells, 2019; 8(5): 426. -


 投稿系统
投稿系统


 下载:
下载: