Ibuprofen treatment for patent ductus arteriosus in preterm infants: a retrospective cohort study in a leading Chinese center
doi: 10.2478/fzm-2021-0013
-
Abstract:
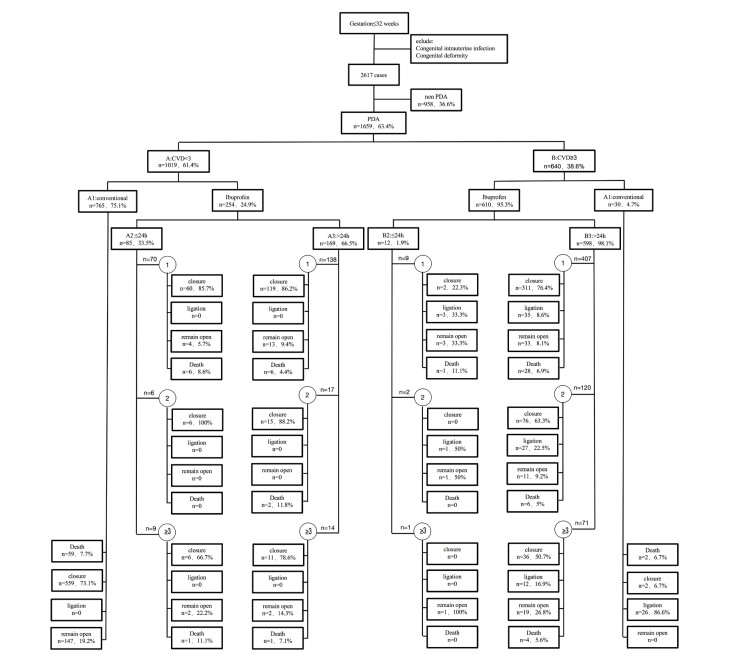
Objective There is a dilemma of ibuprofen treatment with patent ductus arteriosus (PDA) as to how and when to treat. We aimed to clarify this issue in very preterm infants (VPIs; < 32 weeks). Methods: This retrospective study included 1 659 VPIs who were diagnosed with PDA according to echocardiographic examinations and cardiovascular dysfunction scoring system (the CVD scoring). The VPIs were classified into six groups (A1, A2, A3, B1, B2, and B3) based on CVD scores (A, < 3, and B, ≥ 3), and treatment with ibuprofen for PDA (1, conservational management; 2, early ibuprofen treatment; and 3, late ibuprofen treatment). Treatment was stopped when PDA was closed, CVD score was zero or PDA needed ligation. Results: VPIs with CVD scores < 3 had most PDA closure without surgery, and early ibuprofen treatment did not significantly affect PDA closure. VPIs with CVD scores ≥ 3 had some PDA closure after 2 courses of treatment, but closure rates decreased linearly with ibuprofen course (1st 75.2%, 2nd 62.3%, 3rd 50.0%, P < 0.0001), and early ibuprofen treatment (group B2) did not increase PDA closure compared to late ibuprofen treatment (group B3). In these same infants, the longer they were in CVD scores ≥ 3, the more the complications of preterm were increased (retinopathy of prematurity ROP 1st 16.5%, 2nd 23.8%, 3rd 29.6%, P = 0.016; bronchopulmonary dysplasia BPD 1st 15.5%, 2nd 26.7%, 3rd 33.8%, P < 0.0001; intraventricular hemorrhage IVH 1st 20.4%, 2nd 32.4%, 3rd 23.8%, P = 0.015). Conclusion: Ibuprofen is suggested for PDA closure when the PDA reopens or has developed into the stage when the CVD score ≥ 3.
-
Key words:
- preterm infants /
- ibuprofen /
- patent ductus arteriosus /
- cohort study /
- CVD score
-
Table 1. Clinical outcomes according to CVD scores
Variables PDA χ2/t P value CVD < 3 CVD ≥ 3 n=1019 n=640 Surgery(PDA ligation)(n/%) 0 16.5 - - PDA closure (n/%) 75.0(772) 66.5(426) 16.201 < 0.0001 1st course of ibuprofen (n/%) 86.1(179) 75.2(313) 9.439 0.002 2nd course of ibuprofen (n/%) 91.3(21) 62.3(76) 1.136 0.287 3rd course of ibuprofen (n/%) 73.9(17) 50.0(36) 4.041 0.044 RDS (n/%) 66.8(679) 83.5(533) 56.219 < 0.0001 ROP (n/%) 7.7(77) 20.7(131) 59.941 < 0.0001 IVH (n/%) 17.4(179) 25(159) 12.881 < 0.0001 NEC (n/%) 1.1(10) 1.2(8) 0.264 0.607 BPD(n/%) 8.9(90) 22.4(143) 59.539 < 0.0001 Death (n/%) 8.8(75) 6.8(41) 0.538 0.463 PDA closure time (d) 10.8±0.8 14.5±1.4 7.015 < 0.0001 PDA diameter (mm) 2.2±0.5 2.4±0.6 6.280 < 0.0001 Ibuprofen (n/%) 24.9(254) 95.3(610) 779.560 < 0.0001 Ibuprofen after 24h (n/%) 66.5(169) 98.1(598) 178.508 < 0.0001 BPD, pulmonary dysplasia; ROP, retinopathy of prematurity; IVH. intraventricular hemorrhage; NEC, Necrotizing enterocolitis. Table 2. Clinical outcomes according to CVD scores ≥ 3
Variables CVD≥3 B1 vs B2 B1 vs B3 B2 vs B3 B2+B3 vs B1 PDA ligation (%) < 0.0001 < 0.0001 0.053 < 0.0001 (96.7 vs 33.3) (96.7 vs 12.2) (33.3 vs 12.2) (12.6 vs 86.6) PDA closure (%) 0.192 < 0.0001 < 0.001 < 0.0001 (6.7 vs 16.7) (6.7 vs 70.9) (16.7 vs 70.9) (69.8 vs 6.7) ROP (%) 0.730 0.048 0.056 0.057 (34.5 vs 41.7) (34.5 vs 19.4) (41.7 vs 19.4) (19.9 vs 34.5) IVH (%) 0.729 0.010 0.318 0.11 (44.8 vs 33.3) (44.8 vs 23.7) (33.3 vs 23.7) (23.9 vs 44.8) NEC(%) - - - - (0 vs 0) (0 vs 0) (0 vs 1.3) (1.3 vs 0) BPD(%) 0.005 < 0.0001 0.442 < 0.0001 (72.4 vs 25) (72.4 vs 19.9) (25 vs 19.9) (20.0 vs 72.4) Death(%) 1.000 0.947 0.551 1.000 (6.7 vs 8.3) (6.7 vs 6.4) (8.3 vs 6.4) (6.4 vs 6.7) PDA closure time(d) 0.307 0.028 0.554 0.026 (19.2±6.2 vs 11.4±0.2) (19.2±6.2 vs 14.3±1.1) (11.4±0.2 vs 14.3±1.1) (19.2±6.2 vs 14.3±1.1) PDA diameter (mm) 0.622 0.612 0.463 0.625 (2.4±0.5 vs 2.5±0.5) (2.4±0.5 vs 2.3±0.7) (2.5±0.5 vs 2.4±0.7)) (2.4±0.5 vs 2.3±0.7) BPD, pulmonary dysplasia; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; NEC, Necrotizing enterocolitis.
B1, non-ibuprofen intervention; B2, ibuprofen intervention was conducted within 24 h; B3, ibuprofen intervention was conducted after 24h; B2+B3, ibuprofen interventionTable 3. Clinical outcomes according to ibuprofen course and intervention timing
CVD score Time Course ≥3 B2(< 24h) B3(≥24h) 1 2 ≥3 P 1 2 ≥3 P Antenatal 66.7 50 0 - 37.0 39.7 39.4 0.828 Corticosteroid(%) NRDS (%) 66.7 100 100 0.513 79.9 89.3 90.1 0.012 Pneumonia (%) 77.8 100 0 - 29.5 46.7 31.0 0.002 Closure (%) 22.2 0 0 - 76.4 63.3 50.7 < 0.0001 Ligation (%) 33.3 50.0 0 - 8.6 27 16.9 < 0.0001 ROP (%) 44.4 50 0 - 16.5 23.8 29.6 0.016 IVH (%) 11.1 10.0 1 0.018 20.4 32.4 23.8 0.015 NEC (%) 0 0 0 - 1.7 0.8 0 - BPD (%) 33.3 - - - 15.5 26.7 33.8 < 0.0001 Death (%) 11.1 - - - 6.9 5.0 5.6 0.743 RDS, respiratory distress syndrome; BPD, pulmonary dysplasia; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; NEC, Necrotizing enterocolitis.
B2, ibuprofen intervention was conducted within 24 h; B3, ibuprofen intervention was conducted after 24h -
[1] Clyman R I, Couto J, Murphy G M. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Seminars in Perinatology, 2012; 36(2): 123-129. doi: 10.1053/j.semperi.2011.09.022 [2] Hamrick S E G, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics, 2020; 125(5): 1020-1030. http://pdfs.semanticscholar.org/7fee/5f7de84555095946570f66662b4be0f5e36e.pdf [3] Gournay V. The ductus arteriosus: Physiology, regulation, and functional and congenital anomalies. Archives of Cardiovascular Disease, 2011; 104(11): 578-585. doi: 10.1016/j.acvd.2010.06.006 [4] Brown E R. Increased risk of bronchopulmonary dysplasia in infants with patent ductus arterosis. J Pediatr, 1979; 95(5 Pt 2): 865-866. http://www.sciencedirect.com/science?_ob=PdfExcerptURL&_imagekey=1-s2.0-S0022347679804540-main.pdf&_piikey=S0022347679804540&_cdi=272521&_orig=PublicationURL&_zone=rslt_list_item&_fmt=abst&_eid=1-s2.0-S0022347679804540&_issn=00223476&_user=12975512&md5=b93ded76fa26f819f57fa988d0532e62&ie=/excerpt.pdf [5] Hagadorn J I, Brownell E A, Trzaski J M, et al. Trends and variation in management and outcomes of very low birth weight infants with patent ductus arteriosus. Pediatric Research, 2016; 80(6): 785-792. doi: 10.1038/pr.2016.166 [6] Lipman B, Serwer G A, Brazy J E. Abnormal cerebral hemodynamics in preterm infants with patent ductus arteriosus, 1982; 69(6): 778-781. http://www.ncbi.nlm.nih.gov/pubmed/7079043 [7] Sweet D G, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome-2016 Update. Neonatology, 2017; 111(2): 107-125. doi: 10.1159/000448985 [8] Sosenko I R S, Fajardo M F, Claure N, et al. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. Journal of Pediatrics, 2012; 160(6): 929-935. doi: 10.1016/j.jpeds.2011.12.031 [9] Letshwiti J B, Semberova J, Pichova K, et al. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Human Development, 2017; 104: 45-49. doi: 10.1016/j.earlhumdev.2016.12.008 [10] Mohamed M A, El-Dib M, Alqahtani S, et al. Patent ductus arteriosus in premature infants: to treat or not to treat? Journal of Perinatology, 2017; 37(6): 652-657. doi: 10.1038/jp.2017.4 [11] Perez K M, Laughon M M. What is new for patent ductus arteriosus management in premature infants in 2015? Current Opinion in Pediatrics, 2015; 27(2): 158-164. doi: 10.1097/MOP.0000000000000200 [12] Yeh T F, Luken J A, Thalji A, et al. Intravenous indomethacin therapy in premature infants with persistent ductus arteriosus—a double-blind controlled study. J Pediatr, 1981; 98(1): 137-145. doi: 10.1016/S0022-3476(81)80560-4 [13] Yeh T F, Thalji A, Luken L, et al. Improved lung compliance following indomethacin therapy in premature infants with persistent ductus arteriosus. Chest, 1981; 80(6): 698-700. doi: 10.1378/chest.80.6.698 [14] Yeh T F, Raval D, Luken J, et al. Clinical evaluation of premature infants with patent ductus arteriosus: a scoring system with echocardiogram, acid-base, and blood gas correlations. Critical Care Medicine. 1981; 9(9): 655-657. doi: 10.1097/00003246-198109000-00009 [15] Afiune J Y, Singer J M, Leone C R. Echocardiographic post-neonatal progress of preterm neonates with patent ductus arteriosus. Jornal De Pediatria, 2005; 81(6): 454-460. [16] Baraldi E, Filippone M. Chronic lung disease after premature birth. The New England journal of medicine, 2007; 357(19): 1946. doi: 10.1056/NEJMra067279 [17] Mccrea H J, Ment L R. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clinics in Perinatology, 2008; 35(4): 777-792. doi: 10.1016/j.clp.2008.07.014 [18] Thompson A M, Bizzarro M J. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs, 2008; 68(9): 1227-1238. doi: 10.2165/00003495-200868090-00004 [19] Haines L, Fielder A R, Baker H, et al. UK population based study of severe retinopathy of prematurity: screening, treatment, and outcome. Archives of Disease in Childhood Fetal and Neonatal Edition, 2005; 90(3): F240-F244. doi: 10.1136/adc.2004.057570 [20] Sweet D G, Carnielli, V P, Greisen G, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2010 update. Neonatology, 2010; 97(4): 402-417. doi: 10.1159/000297773 [21] Erdeve O, Yurttutan S, Altug N, et al. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Arch dis child fetal neonatal ed, 2012; 97(4): F279-F283. doi: 10.1136/archdischild-2011-300532 [22] Koch J, Hensley G, Rosenfeld C R. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics, 2006; 117(4): 1113-1121. doi: 10.1542/peds.2005-1528 [23] Adrouche-Amrani L, Green R S, Gluck K M, et al. Failure of a repeat course of cyclooxygenase inhibitor to close a PDA is a risk factor for developing chronic lung disease in ELBW infants. BMC Pediatr, 2012; 27(12): 10. doi: 10.1186%2F1471-2431-12-10.pdf [24] Gournay V, Roze J C, Kuster A, et al. Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double-blind, placebocontrolled trial. Lancet, 2004; 364(9449): 1939-1944. doi: 10.1016/S0140-6736(04)17476-X [25] Overmeire B V, Allegaert K, Casaer A, et al. Prophylactic ibuprofen in premature infants: A multicentre, randomised, double-blind, placebocontrolled trial. Journal of Pediatrics, 2004; 364(9449): 1945-1949. http://www.researchgate.net/profile/Alexandra_Casaer/publication/8160478_Overmeire_B_Allegaert_K_Casaer_A_et_al._Prophylactic_ibuprofen_in_premature_infants_a_multicentre_randomised_double-blind_placebo-controlled_trial/links/09e4151487af583ff3000000.pdf [26] Clyman R I. Recommendations for the postnatal use of indomethacin: An analysis of four separate treatment strategies. Journal of Pediatrics, 1996; 128(5 Pt 1): 601-607. http://europepmc.org/abstract/MED/8627430 [27] Ohlsson A, Shah S. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. The Cochrane database of systematic reviews, 2019; 6: CD004213. http://www.uni-kiel.de/aepc/2018/aepcAbstractsFinalPrint/P_244fin.pdf [28] Mitra S, Florez I D, Tamayo M E, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants. Jama the Journal of the American Medical Association, 2018; 319(12): 1221. doi: 10.1001/jama.2018.1896 [29] Overmeire B V, Smets K, Lecoutere D. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. Acc Current Journal Review, 2000; 34310(1): 674-681. doi: 10.1056/nejm200009073431001 [30] Garingo A, Tesoriero L, Cayabyab R, et al. Constitutive IL-10 expression by lung inflammatory cells and risk for bronchopulmonary dysplasia. Pediatric research, 2007; 61(2): 197-202. doi: 10.1203/pdr.0b013e31802d8a1c [31] Van der Linde D, Konings E E, Slager M A, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology, 2011; 58(21): 2241-2247. doi: 10.1016/j.jacc.2011.08.025 [32] Białkowski J, Głowacki J, Zabal C, et al. Patent ductus arteriosus at low and high altitudes: anatomical and haemodynamic features and their implications for transcatheter closure. Kardiologia polska, 2011; 69(5): 431-436. http://www.researchgate.net/profile/Carlos_Zabal/publication/51148776_Patent_ductus_arteriosus_at_low_and_high_altitudes_Anatomical_and_haemodynamic_features_and_their_implications_for_transcatheter_closure/links/0deec515dabfbe3d36000000/Patent-ductus-arteriosus-at-low-and-high-altitudes-Anatomical-and-haemodynamic-features-and-their-implications-for-transcatheter-closure.pdf -


 投稿系统
投稿系统


 下载:
下载: