Tranilast treats cold-related hypertension by reducing the expression of NLRP3 inflammasome
doi: 10.2478/fzm-2021-0012
-
Abstract:
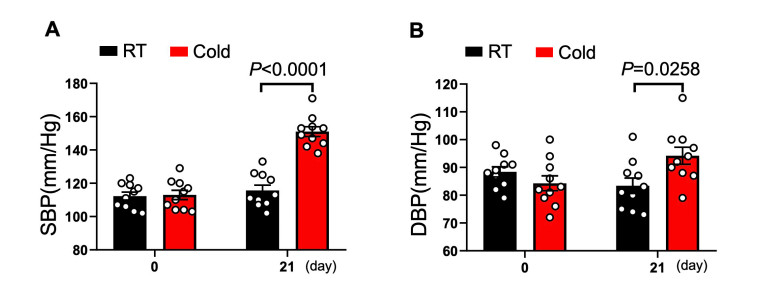
Objective: Cold exposure is associated with increased prevalence of hypertension and the related severe cardiovascular events. Aberrant activation of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome plays an important role in the development of hypertension. Tranilast (TR), an inhibitor of NLRP3, provides a useful pharmacological probe for exploring the role of NLRP3 in pathogenesis associated with inflammation and its potential application as a therapeutic agent. This study was designed to examine the effects of TR on NLRP3 and hypertension in rats exposed to cold environment to simulate the frigid-zone conditions. Methods and results: Sprague Dawley (SD) rats were exposed to moderate cold temperature (4±1℃), and then were randomized to receive TR or vehicle for 3 weeks, while the control group was raised under rat room temperature (RT, 23±1℃). We found that cold exposure substantially increased blood pressure, NLRP3 inflammasome level, and fibrosis in aorta, which were reversed by TR. Conclusion: TR has an anti-hypertensive property in cold environment, and this beneficial action is likely conferred by its inhibitory effects on inflammation and fibrosis. These findings suggest TR as a potential drug for the treatment of cold-induced hypertension.
-
Key words:
- cold exposure /
- blood pressure /
- NLRP3 /
- tranilast
-
Figure 2. Cold exposure increases blood pressure in rats by activating the NLRP3 inflammasome.
(A and B) Western blotting for NLRP3, ASC and Casp1-p20 proteins in aorta isolated from rats in the RT and cold groups. P = 0.031 3, P = 0.012 6 vs. RT group, n = 10 per group. RT, room temperature. (C and D) ELISA for the plasma levels of IL-1β and IL-18 in the RT and cold groups. P = 0.023 8, P = 0.019 4 vs. RT group, n = 10 per group.
Figure 5. Tranilast alleviates aortic fibrosis in cold exposed rats.
(A) Hematoxylin and eosin (H & E) staining (200× magnification; scale bar: 50 μm. (B) Masson's trichrome staining (200× magnification; scale bar: 50 μm). (C) Collagen volume fraction (CVF) per field in the atria of rats from each group. TR, tranilast.
-
[1] Ikäheimo T M. Cardiovascular diseases, cold exposure and exercise. Temperature (Austin), 2018; 5(2): 123-146. doi: 10.1080/23328940.2017.1414014 [2] Huang Y, Jiang H, Chen Y, et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med, 2018; 10(4): e8689. doi: 10.15252/emmm.201708689/pdf [3] Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol, 2009; 27: 229-265 doi: 10.1146/annurev.immunol.021908.132715 [4] Zuurbier C J. NLRP3 Inflammasome in Cardioprotective Signaling. J Cardiovasc Pharmacol, 2019; 74(4): 271-275. doi: 10.1097/FJC.0000000000000696 [5] Sun H J, Ren X S, Xiong X Q, et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis, 2017; 8(10): e3074. doi: 10.1038/cddis.2017.470 [6] Isaji M, Miyata H, Ajisawa Y, et al. Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo. Br J Pharmacol, 1997; 122(6): 1061-1066. doi: 10.1038/sj.bjp.0701493 [7] Yasukawa T, Kimura H, Dong J, et al, Effect of tranilast on proliferation, collagen gel contraction, and transforming growth factor beta secretion of retinal pigment epithelial cells and fibroblasts. Ophthalmic Res, 2002; 34(4): 206-212. doi: 10.1159/000063884 [8] Takahashi A, Taniguchi T, Ishikawa Y, et al. Tranilast inhibits vascular smooth muscle cell growth and intimal hyperplasia by induction of p21(waf1/cip1/sdi1) and p53. Circ Res, 1999; 84(5): 543-550. doi: 10.1161/01.RES.84.5.543 [9] Riteau N, Baron L, Villeret B, et al. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis, 2012; 3(10): e403. doi: 10.1038/cddis.2012.144 [10] Lamkanfi M, Dixit V M. Mechanisms and functions of inflammasomes. Cell, 2014; 157(5): 1013-1022. doi: 10.1016/j.cell.2014.04.007 [11] Jo E K, Kim J K, Shin D M, et al. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol, 2016; 13(2): 148-159. doi: 10.1038/cmi.2015.95 [12] Wang X, Zhao S, Su M, et al. Geraniol improves endothelial function by inhibiting NOX-2 derived oxidative stress in high fat diet fed mice. Biochem Biophys Res Commun, 2016; 474(1): 182-187. doi: 10.1016/j.bbrc.2016.04.097 [13] Chen R, Yin P, Wang L, et al. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ, 2018; 363: k4306. http://www.bmj.com/content/363/bmj.k4306.full.pdf [14] Moghadamnia M T, Ardalan A, Mesdaghinia A, et al. Ambient temperature and cardiovascular mortality: a systematic review and metaanalysis. Peer J, 201; 5: e3574. [15] Crosswhite P, Sun Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension, 2010; 55(6): 1484-1491. doi: 10.1161/HYPERTENSIONAHA.109.146902 [16] Sugama S, Takenouchi T, Fujita M, et al. Cold stress induced morphological microglial activation and increased IL-1β expression in astroglial cells in rat brain. J neuroimmunol, 2011; 233(1-2): 29-36. doi: 10.1016/j.jneuroim.2010.11.002 [17] Oktay A A, Akturk H K, Jahangir E. Diabetes mellitus and hypertension: a dual threat. Curr Opin Cardiol, 2016; 31(4): 402-409. doi: 10.1097/HCO.0000000000000297 [18] Yu J W, Lee M S. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res, 2016; 39(11): 1503-1518. doi: 10.1007/s12272-016-0827-4 [19] Zhang S, Zhang Y, Ahsan M Z, et al. Atorvastatin attenuates coldinduced hypertension by preventing gut barrier injury. J Cardiovasc Pharmacol, 2019; 74(2): 143-151. doi: 10.1097/FJC.0000000000000690 [20] Flaa A, Eide I K, Kjeldsen S E, et al. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension, 2008; 52(2): 336-341. doi: 10.1161/HYPERTENSIONAHA.108.111625 [21] Sun Z. Cardiovascular responses to cold exposure. Front Biosci (Elite Ed), 2010; 2: 495-503. http://europepmc.org/articles/PMC2826836?pdf=render [22] Pasqua T, Pagliaro P, Rocca C, et al. Role of NLRP-3 Inflammasome in Hypertension: A Potential Therapeutic Target. Curr Pharm Biotechnol, 2018; 19(9): 708-714. doi: 10.2174/1389201019666180808162011 [23] Schroder K, Tschopp J. The inflammasomes. Cell, 2010; 140(6): 821-832. doi: 10.1016/j.cell.2010.01.040 [24] Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol, 2011; 41(5): 1196-1202. doi: 10.1002/eji.201141436 [25] Solak Y, Afsar B, Vaziri N D, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res, 2016; 39(8): 567-573. doi: 10.1038/hr.2016.35 [26] Liu D, Zeng X, Li X, et al. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol, 2017; 113(1): 5. doi: 10.1007%2Fs00395-017-0663-9.pdf [27] Zhu Q, Li X X, Wang W, et al. Mesenchymal stem cell transplantation inhibited high salt-induced activation of the NLRP3 inflammasome in the renal medulla in Dahl S rats. Am J Physiol Renal Physiol, 2016; 310(7): 621-627. doi: 10.1152/ajprenal.00344.2015 [28] Krishnan S M, Ling Y H, Huuskes B M, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res, 2019; 115(4): 776-787. doi: 10.1093/cvr/cvy252 [29] Zahid A, Li B, Kombe A J K, et al. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol, 2019; 10: 2538. doi: 10.3389/fimmu.2019.02538 [30] Darakhshan S, Pour A B. Tranilast: a review of its therapeutic applications. Pharmacol Res, 2015; 91: 15-28. doi: 10.1016/j.phrs.2014.10.009 [31] Chen S, Wang Y, Pan Y, et al. Novel Role for Tranilast in Regulating NLRP3 Ubiquitination, Vascular Inflammation, and Atherosclerosis. J Am Heart Assoc, 2020; 9(12): e015513. http://www.researchgate.net/publication/341801014_Novel_Role_for_Tranilast_in_Regulating_NLRP3_Ubiquitination_Vascular_Inflammation_and_Atherosclerosis [32] Zhan C, Bai N, Zheng M, et al. Tranilast prevents doxorubicininduced myocardial hypertrophy and angiotensin Ⅱ synthesis in rats. Life Sci, 2021; 267: 118984. doi: 10.1016/j.lfs.2020.118984 [33] Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant, 2006; 21(4): 850-853. doi: 10.1093/ndt/gfl019 [34] De Miguel C, Rudemiller N P, Abais J M, et al. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep, 2015; 17(1): 507. doi: 10.1007/s11906-014-0507-z -


 投稿系统
投稿系统


 下载:
下载: