Ablation of apoptosis-stimulating of p53 protein 1 protects mice from acute hepatic injury and dysfunction via NF-кB pathway in CCl4-induced hepatotoxicity
doi: 10.2478/fzm-2021-0007
-
Abstract: Acute liver injury (ALI) is characterized by apoptosis, inflammation, and oxidative stress, and pathogenic mechanism of ALI is poorly understood. Apoptosis-stimulating of p53 protein 1 (ASPP1) is involved in environmental responses, tumor growth, and NF-KB activity, which is of critical importance to ALI. However, the role of ASPP1 in ALI remains largely unexplored. The current study aimed to determine the role of ASPP1 in ALI induced by CCl4 and the underlying mechanism. ASPP1 expression was detected in wild type (WT) mice with ALI induced by CCl4. The function of ASPP1 in ALI induced by CCl4 was investigated using conventional knockout ASPP1 mice. ASPP1 expression significantly increased in ALI mice at 24 hours after CCl4 injection. Deletion of ASSP1 ameliorated apoptosis, inflammation, and necrosis in ALI relative to WT mice. In addition, deficiency of ASPP1 improved liver flood flow as well as ALT and AST levels. The levels of phosphorylated p65 and phosphorylated IκBα were lower in ASPP1-/- mice than in WT mice with ALI. These results implicate that deletion of ASPP1 may act via inhibition of the NF-кB pathway and protect mice from ALI, which may be a new potential therapeutic target for the treatment of ALI.
-
Key words:
- Apoptosis /
- ASPP1 /
- CCl4 /
- Liver injury /
- Inflammation /
- NF-κB
-
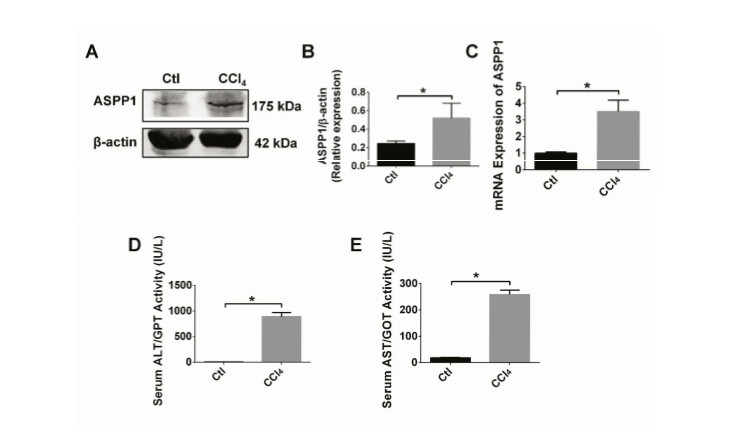
Figure 1. Expression profiles of ASPP1 and liver injury markers in wild type mice with CCl4-induced acute hepatic injury.
A, B. Protein expression of ASPP1 in liver tissue of mice 24 hrs after treatment with 5% CCl4 (intraperitoneal injection; dose: 2µL/g body weight) injection. n = 3; *P < 0. 05. C. mRNA expression of ASPP1 in liver tissue of mice 24 hrs after treatment with 5% CCl4 (ip; 2µL/g body weight). n = 3; *P < 0. 05. D, E. Serum ALT/AST activity in liver tissue of mice 24 hrs after treatment with 5% CCl4 (ip; 2µL/g body weight). n = 3; *P < 0. 05. CTL, Control; Hrs, hours; IU, international units; L, litter.
Figure 2. Ablation of ASPP1 alleviates CCl4-induced acute liver injury in wild-type and ASPP knockout mice.
A, B. Protein expression of ASPP1 in WT and ASPP1 knockout (ASPP1-/-) mice. n = 4; *P < 0.05. C. mRNA expression of ASPP1 in WT and ASPP1-/- mice. n =7; *P < 0. 05. D. Comparison of morphological alterations of the livers between WT and ASPP1-/- mice without or with CCl4 treatment 24 hrs post-injection. E, F. Representative images (E) and statistical data on blood flow rate (volume/minute) in the liver. n = 3; *P < 0. 05. Upper panels of E show blood perfusion to liver tissue with red, yellow and blue colors indicating high, moderate and low blood flow rates, respectively. Middle and bottom panels of E display the red-line outlined areas indicating the selected regions for the measurements of blood flow. G. Representative images of Hematoxylin and Eosin (H&E) staining (scale bar = 100µm) showing the histological alterations of liver tissue with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. The outlined area indicates necrosis tissue, and the arrows indicate balloon-like degeneration caused by CCl4. H. Statistical analysis of zone of hepatocellular necrosis grading[39]. n = 9; *P < 0. 05. CTL, control; WT, wild type; KO, knockout; ns, no significance.
Figure 3. Ablation of ASPP1 reduces the levels of liver injury markers and increases the levels of antioxidants.
A, B. Serum levels of ALT and AST with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. ALT, n = 8; AST, n =7, *P < 0. 05. C. Serum level of SOD with and without CCl4 treatment. n = 5; *P < 0. 05. ALT, alanine transaminase; AST, aspartate aminotransferase; SOD, superoxide dismutase.
Figure 4. Ablation of ASPP1 reduces apoptosis in mice with acute liver injury.
A. Representative images of TUNEL staining with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. scale bar = 20µm. Arrows point to the TUNEL-positive cells. B. Statistical data on TUNEL-positive cells. n = 3; *P < 0.05.
C, D. Protein expression of cleaved caspase 3 with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 4; *P < 0.05. E, F. Protein expression of Bax before and after CCl4 treatment. n = 4; *P < 0.05. G. The mRNA of expression of Bax with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 4; *P < 0.05. H, I. Protein expression of BCL-2 with comparisons between WT and ASPP1-/- mice with and without CCl4 treatment. n = 4; *P < 0.05. J. The mRNA expression of BCL-2 with comparisons between WT and ASPP1-/- mice with and without CCl4 treatment. n = 3; *P < 0.05. DAPI, 4', 6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase mediate dUTP nick end labeling; β-actin, beta actin; Bax, BCL-2-associated X protein; BCL-2, B-cell lymphoma 2.Figure 5. Ablation of ASPP1 lessens inflammation in mice with acute liver injury.
A. mRNA expression of IL-1β with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 3; *P < 0.05. B. mRNA expression of IL-6 with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 3; *P < 0.05. C. mRNA expression of TLR4 with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 5; *P < 0.05. D. mRNA expression of TNF-α with comparisons between WT and ASPP1-/- mice with and withoutCCl4 treatment. n = 4; *P < 0.05. E. mRNA expression of ICAM1 with comparisons between WT and ASPP1-/- mice with and without CCl4 treatment. n = 3; *P < 0.05. IL-1β, interleukin 1 beta; IL-6, interleukin 6; TLR4, Toll like receptors 4; TNF-α, tumor necrosis factor alpha; ICAM1, intercellular adhesion molecule 1.
Figure 6. Effect of IκBα/NF–κB in mice with acute liver injury.
A, B, C. Protein levels of IκBα and p-IκBα and their respective statistical data with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 4; *P < 0.05. D, E, F. Protein levels of NF-κB/P65, p-NF-κB/P65 and their statistical analysis with comparisons between WT and ASPP1-/-mice with and without CCl4 treatment. n = 4; *P < 0.05. IκBα, inhibitor of kappa B alpha (inhibitor protein of NF-κB); p-IκBα, phosphorylated inhibitor of kappa B alpha; NF-κB/P65, nuclear factor kappa B/P65; p-NF-κB/P65, phosphorylated nuclear factor kappa B/P65; β-actin, beta actin.
Table 1. Information on the antibodies used for western blot analysis
Antibody Origin Dilutions Cat. Number Molecular weight Company ASPP1 Rabbit 1:1000 A4355 175kDa Sigma Aldrich Bax Mouse 1:1000 60267-1-1g 23 kDa Protein Tech Bcl-2 Rabbit 1:1000 0712019 26 kDa Cell Signaling Cleaved Caspase 3 Rabbit 1:1000 9664S 19 kDa Cell Signaling IKBα Mouse 1:1000 4814 39 kDa Cell Signaling p- IKBα Rabbit 1:1000 2859 39 kDa Cell Signaling NF-KB P65 Rabbit 1:1000 8242 65 kDa Cell Signaling p- NF-KB P65 Rabbit 1:1000 3033 65 kDa Cell Signaling β-actin Mouse 1:20000 66009-1-1g 42 kDa Protein Tech ASPP1, Apoptosis-stimulating of p53 protein 1; Bax, BCL-2-associated X protein; BCL-2, B-cell lymphoma 2(40); IKBα, Inhibitor of kappa B alpha (inhibitor protein of NF-KB); p-IKBα, phosphorylated inhibitor of kappa B alpha; NF-KB P65, Nuclear factor kappa B P65; p-NF-KB P65, phosphorylated Nuclear factor kappa B P65(18); β-actin, beta actin. Table 2. Sequences of the Primer Pairs used for qRT-PCR
Gene Sense (5'-3') Antisense (5'-3') ASPP1 ATGATATTAACCGTGTTCTTG CACTTCCTCTCCACACTTCAGC Bax TGGAAGAAGATGGGCTGAGG TTCCCACCCCTCCCAATAAT Bcl-2 AACGATTGTGGCAGTCCCTT GAAGTGCTCAGGTGCCATCT β-actin GACAGCAGTTGGTTGGAGCA TTGGGAGGGTGAGGGACTTC TNF-α CTGAACTTCGGGGTGATCGG GGCTTGTCACTCGAATTTTGAGA IL-6 CTGCAAGAGACTTCCATCCAG AGTGGTATAGACAGGTCTGTTGG IL-1β GAAATGCCACCTTTTGACAGTG TGGATGCTCTCATCAGGACAG ICAM-1 TGCCTCTGAAGCTCGGATATAC TCTGTCGAACTCCTCAGTCAC TLR4 ATGGCATGGCTTACACCACC GAGGCCAATTTTGTCTCCACA ASPP1, Apoptosis-stimulating of p53 protein 1; Bax, BCL-2-associated X protein; BCL-2, B-cell lymphoma 2; β-actin, Beta actin; TNF-α, Tumor necrosis factor alpha; IL-6, Interleukin 6; IL-1β, Interleukin 1beta; ICAM1, Intercellular adhesion Molecule; TLR4, Toll like receptors 4. -
[1] Piñeiro-Carrero V M, Piñeiro E O. Liver. Pediatrics, 2004; 113(4 Suppl): 1097-1106. [2] Ippolito J A, Curtis B J, Choudhry M A, et al. Alcohol and immunology: Summary of the 2012 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol (Fayetteville, NY), 2013; 47(8): 589-593. doi: 10.1016/j.alcohol.2013.09.003 [3] Shen H, Jiang L, Lin J D, et al. Brown fat activation mitigates alcohol-induced liver steatosis and injury in mice. The Journal of Clinical Investigation, 2019; 129(6): 2305-2317. doi: 10.1172/JCI124376 [4] Yoda T, Crawshaw L I, Saito K, et al. Effects of alcohol on autonomic responses and thermal sensation during cold exposure in humans. Alcohol (Fayetteville, NY), 2008; 42(3): 207-212. doi: 10.1016/j.alcohol.2008.01.006 [5] Lopez A M, Hendrickson R G. Toxin-induced hepatic injury. Emergency Medicine Clinics of North America, 2014; 32(1): 103-25. doi: 10.1016/j.emc.2013.09.005 [6] Alam M F, Safhi M M, Anwer T, et al. Therapeutic potential of Vanillylacetone against CCl(4) induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines and apoptosis in Swiss albino mice. Experimental And Molecular Pathology, 2018; 105(1): 81-88. doi: 10.1016/j.yexmp.2018.06.001 [7] Alric L, Orfila C, Carrere N, et al. Reactive oxygen intermediates and eicosanoid production by kupffer cells and infiltrated macrophages in acute and chronic liver injury induced in rats by CCl4. Inflammation Research: Official Journal of The European Histamine Research Society, 2000; 49(12): 700-707. doi: 10.1007/s000110050649 [8] Sullivan A, Lu X. ASPP: a new family of oncogenes and tumour suppressor genes. British Journal of Cancer, 2007; 96(2): 196-200. doi: 10.1038/sj.bjc.6603525 [9] Samuels-Lev Y, O'Connor D J, Bergamaschi D, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Molecular Cell, 2001; 8(4): 781-794. doi: 10.1016/S1097-2765(01)00367-7 [10] Aylon Y, Ofir-Rosenfeld Y, Yabuta N, et al. The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1. Genes & development, 2010; 24(21): 2420-2429. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2964752/pdf/2420.pdf?origin=publication_detail [11] Agirre X, Román-Gómez J, Jiménez-Velasco A, et al. ASPP1, a common activator of TP53, is inactivated by aberrant methylation of its promoter in acute lymphoblastic leukemia. Oncogene, 2006; 25(13): 1862-1870. doi: 10.1038/sj.onc.1209236 [12] Xu P, Yao J, Ji J, et al. Deficiency of apoptosis-stimulating protein 2 of p53 protects mice from acute hepatic injury induced by CCl(4) via autophagy. Toxicology Letters, 2019; 316: 85-93. doi: 10.1016/j.toxlet.2019.09.006 [13] Baker R G, Hayden M S, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metabolism, 2011; 13(1): 11-22. doi: 10.1016/j.cmet.2010.12.008 [14] Hayden M S, Ghosh S. Shared principles in NF-kappaB signaling. Cell, 2008; 132(3): 344-362. doi: 10.1016/j.cell.2008.01.020 [15] Li B, Li D, Wang Y, et al. Schisantherin A alleviated alcohol-induced liver injury by the regulation of alcohol metabolism and NF-kB pathway. Experimental Animals, 2018; 67(4): 451-461. doi: 10.1538/expanim.18-0021 [16] Zhang Z, Tian L, Jiang K. Propofol attenuates inflammatory response and apoptosis to protect d-galactosamine/lipopolysaccharide induced acute liver injury via regulating TLR4/NF-κB/NLRP3 pathway. International Immunopharmacology, 2019; 77: 105974. doi: 10.1016/j.intimp.2019.105974 [17] Li Z, Zhang J, Mulholland M, et al. mTOR activation protects liver from ischemia/reperfusion-induced injury through NF-κB pathway. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 2017; 31(7): 3018-3026. doi: 10.1096/fj.201601278R [18] Luo P, Qin C, Zhu L, et al. Ubiquitin-Specific peptidase 10 (USP10) inhibits hepatic steatosis, insulin resistance, and inflammation through sirt6. Hepatology (Baltimore, Md), 2018; 68(5): 1786-803. doi: 10.1002/hep.30062 [19] Hua H, Zhang Z, Qian Y, et al. Inhibition of the mitochondrial complex-1 protects against carbon tetrachloride-induced acute liver injury. Biomedicine & Pharmacotherapy= Biomedecine & Pharmacotherapie, 2019; 115: 108948. http://www.ncbi.nlm.nih.gov/pubmed/31078037 [20] Boll M, Weber L W, Becker E, et al. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Zeitschrift fur Naturforschung C, Journal of Biosciences, 2001; 56(7-8): 649-659. http://www.onacademic.com/detail/journal_1000037959494510_5410.html [21] Hershko C, Abrahamov A, Konijn AM, et al. Objectives and methods of iron chelation therapy. Bioinorganic Chemistry and Applications, 2003; 1(2): 151-68. doi: 10.1155/S1565363303000128 [22] Liu H, Wang Z, Nowicki M J. Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice. World Journal of Gastroenterology, 2014; 20(48): 18189-18198. doi: 10.3748/wjg.v20.i48.18189 [23] Dufour D R, Lott J A, Nolte F S, et al. Diagnosis and monitoring of hepatic injury. Ⅱ. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clinical Chemistry, 2000; 46(12): 2050-2068. doi: 10.1093/clinchem/46.12.2050 [24] Wu L, Zhang Q, Mo W, et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Scientific Reports, 2017; 7(1): 9289. doi: 10.1038/s41598-017-09673-5 [25] Feng J, Wang C, Liu T, et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis, 2019; 23(9): 6479-6493. [26] Takahashi H, Shigefuku R, Yoshida Y, et al. Correlation between hepatic blood flow and liver function in alcoholic liver cirrhosis. World Journal of Gastroenterology, 2014; 20(45): 17065-17074. doi: 10.3748/wjg.v20.i45.17065 [27] Van der Graaff D, Kwanten W J, Francque S M. The potential role of vascular alterations and subsequent impaired liver blood flow and hepatic hypoxia in the pathophysiology of non-alcoholic steatohepatitis. Medical Hypotheses, 2019; 122: 188-197. doi: 10.1016/j.mehy.2018.11.014 [28] Zhao J, Wu G, Bu F, et al. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region-containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology (Baltimore, Md), 2010; 51(1): 142-153. doi: 10.1002/hep.23247 [29] Liu H, Chen F, Zhang L, et al. A novel all-trans retinoic acid derivative 4-amino-2-trifluoromethyl-phenyl retinate inhibits the proliferation of human hepatocellular carcinoma HepG2 cells by inducing G0/G1 cell cycle arrest and apoptosis via upregulation of p53 and ASPP1 and downregulation of iASPP. Oncology Reports, 2016; 36(1): 333-341. doi: 10.3892/or.2016.4795 [30] Zhao K, Yu M, Zhu Y, et al. EGR-1/ASPP1 inter-regulatory loop promotes apoptosis by inhibiting cyto-protective autophagy. Cell Death & Disease, 2017; 8(6): e2869. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5520923/pdf/cddis2017268a.pdf [31] Rasool F, Nayak D, Katoch A, et al. Regiospecific synthesis of ring a fused withaferin a isoxazoline analogues: induction of premature senescence by W-2b in proliferating cancer cells. Scientific Reports, 2017; 7(1): 13749. doi: 10.1038/s41598-017-13664-x [32] Chen L, Li S, Guo X, et al. The role of GSH in microcystin-induced apoptosis in rat liver: Involvement of oxidative stress and NF-κB. Environmental toxicology, 2016; 31(5): 552-560. http://dc.cern.ac.cn/downloadAttachment?filename=2016/DHL/6.pdf [33] Zhao X, Jiang K, Liang B, et al. Anticancer effect of xanthohumol induces growth inhibition and apoptosis of human liver cancer through NF-κB/p53-apoptosis signaling pathway. Oncology Reports, 2016; 35(2): 669-675. doi: 10.3892/or.2015.4455 [34] Chen X, Zhang J, Yi R, et al. Hepatoprotective effects of lactobacillus on carbon tetrachloride-induced acute liver injury in mice. International Journal of Molecular Sciences, 2018; 19(8). http://www.onacademic.com/detail/journal_1000040537775610_188e.html [35] Qi B, Zhang S, Guo D, et al. Protective effect and mechanism of ginsenoside Rg1 on carbon tetrachloride-induced acute liver injury. Molecular Medicine Reports, 2017;16(3): 2814-22. doi: 10.3892/mmr.2017.6920 [36] Benyamini H, Friedler A. The ASPP interaction network: electrostatic differentiation between pro- and anti-apoptotic proteins. Journal of molecular recognition: JMR, 2011; 24(2): 266-274. doi: 10.1002/jmr.1048 [37] Feldman A T, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods in Molecular biology (Clifton, NJ), 2014;1180: 31-43. doi: 10.1007%2F978-1-4939-1050-2_3.pdf [38] Slaoui M, Bauchet A L, Fiette L. Tissue sampling and processing for histopathology evaluation. Methods in Molecular Biology (Clifton, NJ), 2017; 1641: 101-114. http://europepmc.org/abstract/MED/28748459 [39] Camargo C A Jr., Madden J F, Gao W, et al. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology (Baltimore, Md), 1997; 26(6): 1513-1520. doi: 10.1002/hep.510260619 [40] Chen W B, Lai S S, Yu D C, et al. GGPPS deficiency aggravates CCl4-induced liver injury by inducing hepatocyte apoptosis. Febs Letters, 2015; 589(10): 1119-1126. doi: 10.1016/j.febslet.2015.03.015 -


 投稿系统
投稿系统


 下载:
下载: