Tridepsides from the endophytic fungus colletotrichum gloeosporioides associated with a toxic medicinal plant tylophora ovata
doi: 10.2478/fzm-2021-0006
-
Abstract:
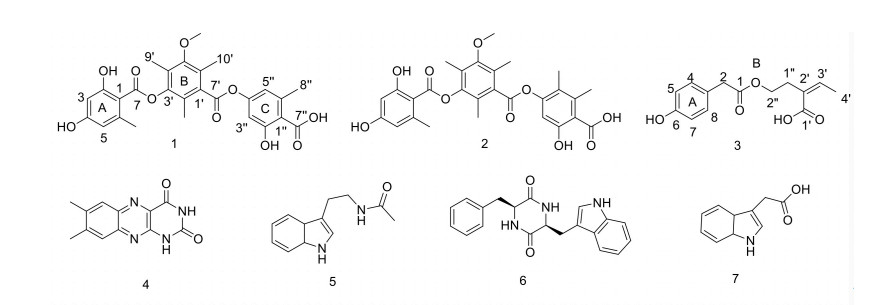
Introduction Bioactive secondary metabolites from the microbes living in frigid, toxic or other extreme environments are emerging as a new medicinal resource. Here, we report the discovery of new antidiabetic and anti-inflammatory compounds with novel structures from endophytic fungi hosted toxic medicinal plant. Methods The endophytic fungus isolated from toxic plants was fermented and extracted. The obtained extracts were purified with preparative HPLC to yield pure compounds. The purified compounds were examined by PTP1b inhibition and NO inhibition assays to evaluate their bioactivities. Results One new tridepsides (Compound 1), one new benzeneacetic acid derivative (Compound 3) and five known compounds (Compounds 2 and 4-7) were isolated from the ethyl acetate extract of Colletotrichum gloeosporioides, an endophytic fungus obtained from a toxic medicinal plant Tylophora ovata. Their structures were determined by spectroscopic data (1D and 2D NMR, HRESIMS) analyses. Compound 2 showed significant inhibitory activity against PTP1b with an IC50 value of 0.84 μM. Compounds 2 and 3 exhibited moderate inhibitory activities against the NO (nitric oxide) release in LPS-induced RAW 264.7 cells at 10 μM with percent inhibition of 39% and 33%, respectively. Conclusion The Compound 2 has potent PTP1b inhibitory effect indicating its antidiabetic potential and thus might be considered a lead compound for antidiabetic drug development. -
Key words:
- endophytic fungus /
- toxic medicinal plants /
- tridepsides /
- antidiabetic /
- anti-inflammation
-
Table 1. NMR data (600 MHz, methanol-d4) on Compound 1
Position δH (J in Hz) δC δH (J in Hz) δC 1 104.5, C 7' 167.2, C 2 165.0, C 8' 2.13 s 13.1, CH3 3 6.26 d (1.94) 102.0, CH 9' 2.15 s 10.3, CH3 4 164.9, C 10' 2.36 s 16.8, CH3 5 6.35 d (1.94) 113.2, CH 1" 113.2, C 6 145.0, C 2" 165.0, C 7 167.6, C 3" 6.70 d (2.30) 109.0, CH 8 2.65 s 24.8, CH3 4" 155.6, C 1' 134.0, C 5" 6.66 d (2.30) 116.8, CH 2' 127.1, C 6" 144.8, C 3' 150.9, C 7" 170.7, C 4' 123.3, C 8" 2.61 s 23.7, CH3 5' 155.2, C -OCH3 3.86 s 62.8, CH3 6' 127.8, C Table 2. NMR data (600 MHz) of Compound 3 in methanol-d4
Position δHa(J in Hz) δCb 1 173.8, C 2 3.53 s 41.2, CH2 3 126.2, C 4/8 7.08 d, (8.0) 131.3 CH2 5/7 6.74 d, (8.0) 116.3, 6 CH2 157.6, C 1' 169.8, C 2' 156.9, C 3' 5.69 q (1.3) 119.0, 4' 2.14 d (1.3) CH 1" 2.49 ddd (6.4, 6.4, 1.1) 18.6, CH3 2" 40.6, CH2 4.27 t (6.4) 63.2, CH2 Table 3. The anti-inflammatory activity of Compounds 2 and 3
Compound Anti-inflammatory activity (10 μM) Inhibition rate of NO production (%) Inhibition rate of Cell proliferation (%) 2 39.34% -10.84% 3 33.04% 35.92% DEX (dexamethasone) 99.99% 2.89% -
[1] Liu Y B, Ding G Z, Li Y, et al. Structures and absolute configurations of penicillactones A–C from an endophytic microorganism, penicillium dangeardii pitt. Org. Lett, 2013; 15: 5206-5209. doi: 10.1021/ol4023485 [2] Liu Y B, Li Y, Qu J, et al. Eremophilane sesquiterpenes and polyketones produced by an endophytic guignardia fungus from the toxic plant gelsemium elegans. J. Nat. Prod, 2015; 78: 2149-2154. doi: 10.1021/np5009027 [3] Liu J Y, Zhao X, Liang X X, et al. Dothiorelone derivatives from an endophyte diaporthe pseudomangiferaea inhibit the activation of human lung fibroblasts MRC-5 cells. Fitoterapia, 2018; 127: 7-14. doi: 10.1016/j.fitote.2018.04.009 [4] Liu Y B, Li Y, Liu Z, et al. Sesquiterpenes from the endophyte glomerella cingulata. J. Nat. Prod, 2017; 80: 2609-2614. doi: 10.1021/acs.jnatprod.7b00054 [5] Liu Z, Zhao J Y, Sun S F, et al. Sesquiterpenes from an endophytic aspergillus flavus. J. Nat. Prod, 2019; 82: 1063-1071. doi: 10.1021/acs.jnatprod.8b01084 [6] Zou W X, Meng J C, Lu H, et al. Metabolities of colletorichum gloeosrioides, an endophytic fungus in Artemisia mongolica, J. Nat. Prod, 2000; 63: 1529-1530. doi: 10.1021/np000204t [7] Bang S, Lee C, Kim S, et al. Neuroprotective glycosylated cyclic lipodepsipeptides, Colletotrichamides A-E, from a halophyte-associated fungus, Colletotrichu-m gloeosporioides JS419. J. Org. Chem, 2019; 84: 10999-11006. doi: 10.1021/acs.joc.9b01511 [8] Luo Y P, Zheng C J, Chen G Y, et al. Three new polyketides from a mangrove-derived fungus Colletotrichum gloeosporioides. J. Antibiot, 2019; 72: 513-517. doi: 10.1038/s41429-019-0178-8 [9] Yang Z D, Li Z J, Zhao J W, et al. Secondary metabolites and PI3K inhibitory activity of Colletotrichum gloeosporioides, a fungal endophyte of Uncaria rhynchophylla. Curr. Microbiol, 2019; 76: 904-908. doi: 10.1007/s00284-019-01707-7 [10] Pamela N K, Sergi H A, Armelle T T, et al. Augustin, Colletotrin: a sesquiterpene lactone from the endophytic fungus Colletotrichum gloeosporioides associated with Trichilia monadelpha. J. Chem. Sci, 2017; 72: 697-703. http://www.degruyter.com/view/j/znb.2017.72.issue-10/znb-2017-0058/znb-2017-0058.xml?format=INT [11] Vanessa M C, Maria L Z, Ioanis H L, et al. Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champac. Molecules, 2014; 19: 19243-19252. doi: 10.3390/molecules191119243 [12] Inacio M L, Silva G H, Teles H L, et al. Antifungal metabolites from Colletotrichum gloeosporioides, an endophytic fungus in Cryptocarya mandioccana Nees (Lauraceae). Biochem. Syst. Ecol, 2006; 34: 822-824. doi: 10.1016/j.bse.2006.06.007 [13] Liu H X, Tan H B, Chen Y C, et al. Secondary metabolites from the colletotrichum gloeosporioides A12, an endophytic fungus derived from Aquilaria sinensis. Nat. Prod. Rep, 2018; 32: 2360-2365. doi: 10.1080/14786419.2017.1410810 [14] Andre A, Wojtowicz N, Toure K, et al. New acorane sesquiterpenes isolated from the endophytic fungus Colletotrichum gloeosporioides SNB-GSS07. Tetrahedron. Lett, 2017; 58: 1269-1272. doi: 10.1016/j.tetlet.2017.02.024 [15] Chen X W, Yang Z D, Sun J H, et al. Colletotrichine A, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Rep, 2018; 32: 880-884. doi: 10.1080/14786419.2017.1365071 [16] Li Y, Wei W, Wang R L, et al. Colletolides A and B, two new γ-butyrolactone derivatives from the endophytic fungus Colletotrichum gloeosporioide. Phytochem. Lett, 2019; 33: 90-93. doi: 10.1016/j.phytol.2019.08.004 [17] Zhao J Y, Wang X J, Liu Z, et al. Nonadride and spirocyclic anhydride derivatives from the plant endophytic fungus Talaromyces purpurogenus. J. Nat. Prod, 2019; 82: 2953-2962. doi: 10.1021/acs.jnatprod.9b00210 [18] Huneck S, Porzel A, Schmidt J, et al. A tridepside from Umbilicaria crustulosa. Phytochemistry, 1993; 32: 475-477. doi: 10.1016/S0031-9422(00)95022-2 [19] Zou W X, Meng J C, Lu H, et al. Metabolites of colletotrichum gloeosporioides, an endophytic fungus in artemisia mongolica. J. Nat. Prod, 2000; 63: 1529-1530. doi: 10.1021/np000204t [20] Gao T, Cao F, Yu H, et al. Secondary metabolites from the marine fungus Aspergillus sydowii. Chem. Nat. Compd, 2017; 53: 1204-1207. doi: 10.1007/s10600-017-2241-7 [21] Pedras M S, Yu Y, Liu J, et al. Metabolites produced by the phytopathogenic fungus Rhizoctonia solani: isolation, chemical structure determination, syntheses and bioactivity. Ztschrift. für Naturforschung, 2005; 60: 9-10. http://www.degruyter.com/dg/viewarticle.fullcontentlink:pdfeventlink/$002fj$002fznc.2005.60.issue-9-10$002fznc-2005-9-1010$002fznc-2005-9-1010.pdf/znc-2005-9-1010.pdf?t:ac=j$002fznc.2005.60.issue-9-10$002fznc-2005-9-1010$002fznc-2005-9-1010.xml [22] Gubiani J R, Teles H L, Silva G H, et al. Cyclo-(TRP-PHE) diketopiperazines from the endophytic fungus Aspergillus versicolor isolated from Piper aduncum. Quim. Nova, 2017; 40: 138-142. http://quimicanova.sbq.org.br/audiencia_pdf.asp?aid2=6521&nomeArquivo=AR20160256.pdf [23] Evidente A, Iacobellis N S, Sisto A. Isolation of indole-3-acetic acid methyl ester, a metabolite of indole-3-acetic acid from Pseudomonas amygdali. Experientia, 1993; 49: 182-183. doi: 10.1007/BF01989428 [24] Zucconi L, Canini F, Temporiti M E, et al. Extracellular Enzymes and Bioactive Compounds from Antarctic Terrestrial Fungi for Bioprospecting. Int. J. Env. Res. Pub. He, 2020; 17: 6459. doi: 10.3390/ijerph17186459 [25] Eleftheriou P, Geronikaki A, Petrou A. PTP1b Inhibition, A Promising Approach for the Treatment of Diabetes Type Ⅱ. Curr. Top. Med. Chem, 2019; 19: 246-263. doi: 10.2174/1568026619666190201152153 [26] Sharma B, Xing L X, Yang F, et al. Recent advance on PTP1B inhibitors and their biomedical applications. Eur. J. Med. Chem, 2020; 199: 112376. doi: 10.1016/j.ejmech.2020.112376 -


 投稿系统
投稿系统


 下载:
下载: