ISG15 promotes M5-induced hacat cell proliferation through Wnt signaling in psoriasis
doi: 10.1515/fzm-2024-0022
-
Abstract:
Objective Psoriasis is a common chronic, recurrent, immune-mediated inflammatory skin disease, which tends to occur in cold areas. Its pathogenesis is currently unclear. This study aims to screen differentially expressed genes in the psoriasis dataset, identify the central genes, detect the expression of central genes in psoriasis lesions of patients in the cold regions and then conduct further research. Methods Differential genes associated with psoriasis in the GEO database were analyzed, and functional enrichment analysis and protein-protein interaction network analysis. The expression results of the identified genes were validated in psoriasis cell models. The ISG15 gene, which showed the most significant difference in expression, was further studied. The expression level of ISG15 protein in psoriasis was examined. Then, we knocked out ISG15 in psoriasis cell models and detected keratinocyte proliferation by MTT, Real-Time PCR and Western Blot. Western Blot showed the expression of β-catenin after ISG15 gene knockout. Results We detected the protein expression of ISG15 in the cold area of Northeast China, and found that the expression of ISG15 increased in patients with psoriasis, and the proliferation of keratinocytes and the expression of β-catenin decreased in psoriasis cell model after ISG15 was knocked down. ISG15 regulates keratinocyte proliferation through Wnt signaling pathway in psoriasis. Conclusions ISG15 expression is increased in psoriatic cells and skin lesions of patients with psoriasis. In psoriasis, ISG15 promotes keratinocyte proliferation through the Wnt signaling pathway. -
Key words:
- psoriasis /
- keratinocyte proliferation /
- ISG15 /
- Wnt signaling pathway
-
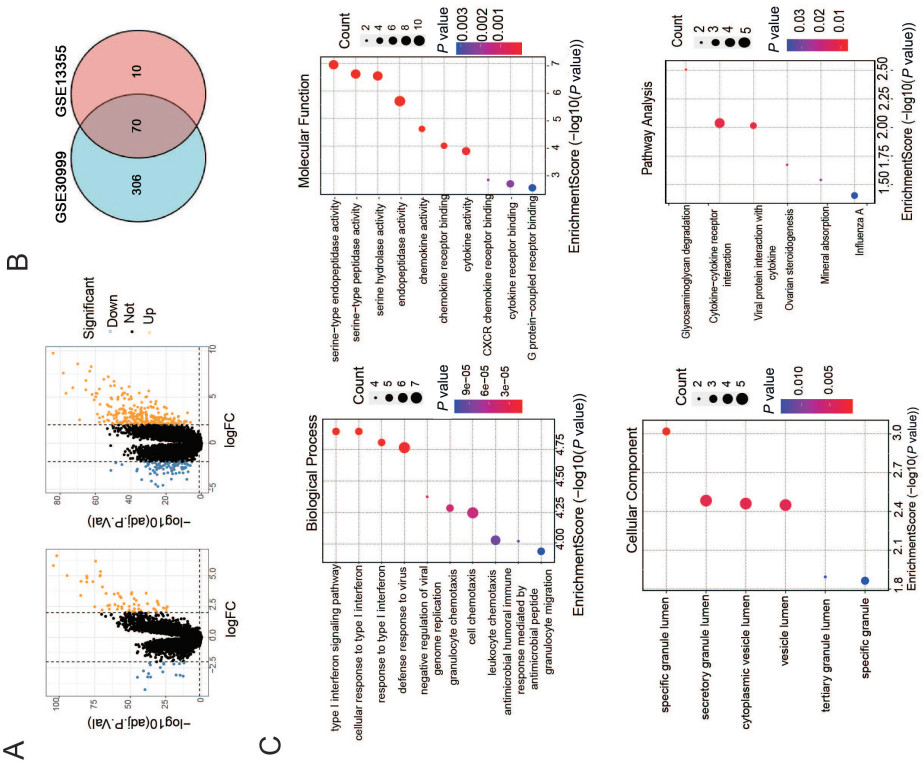
Figure 1. GO and KEGG enrichment analyses of differentially expressed genes derived fromGSE13355 and GSE30999 datasets
(A) Volcano map showing differentially expressed genes in GSE13355 and GSE30999 datasets. Orange color indicates high expression and blue low expression. (B) Venn diagram of the common differentially expressed genes (cross area) of GSE13355 and GSE30999 datasets. (C) Bubble map illustrating GO analysis (including biological process, cell component, and molecular function) and KEGG enrichment analysis of differentially expressed genes.
Figure 2. Protein-protein interaction (PPI) networks of differentially expressed genes and real-time PCR quantification of expression levels of key genes
(A) PPI networks of differentially expressed genes. The size of the circle is proportional to the value. (B) Real time-PCR quantification of the expression levels of ISG15, LTF, and RSAD2 genes in psoriasis patients. **P < 0.01.
Figure 4. Silencing ISG15 in a psoriatic cell model and verifying its phenotypic proliferation efficiency
(A) Western blot images showing ISG15 protein expression after transfection with NC-siRNA or siISG15. (B) Statistical data of western blot bands. Note that silencing ISG15 reduced psoriatic cell proliferation. (C) Psoriatic cell proliferation rate following ISG15 knockdown determined by the MTT assays conducted on 1, 2, 3, 4 and 5 days after siISG15 transfection. **P < 0.01.
Figure 5. Silencing ISG15 reduces the expression of Wnt signaling and regulates keratinocyte proliferation in psoriasis
(A) Western blot images depicting the changes of protein levels of β-catenin post-ISG15 silence. (B) Statistical data of Western blot bands for β-catenin. (C) Psoriatic cell proliferation rate with ISG15 knockdown was measured by MTT assay at 1, 2, 3, 4 and 5 days. (D) Western blot images showing the alterations of protein levels of β-catenin and Cyclin D1, a key regulator of cell cycle. (E-F) Statistics of Western blot bands. **P < 0.01.
-
[1] Mease P, Palmer J, Hur P, et al. Utilization of the validated psoriasis epidemiology screening tool to identify signs and symptoms of psoriatic arthritis among those with psoriasis: a cross-sectional analysis from the US-based corrona psoriasis registry. J Eur Acad Dermatol Venereol, 2019; 33(5): 886-892. doi: 10.1111/jdv.15443 [2] De Simone C, Caldarola G, Moretta G, et al. Moderate-to-severe psoriasis and pregnancy: impact on fertility, pregnancy outcome and treatment perspectives. G Ital Dermatol Venereol, 2019; 154(3): 305-314. [3] Parisi R, Iskandar I, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ, 2020; 369: m1590. [4] Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci, 2020; 21(20): 7488. doi: 10.3390/ijms21207488 [5] Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/ T 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol, 2017, 31(10): 1616-1626. doi: 10.1111/jdv.14433 [6] Hawkes J E, Chan T C, Krueger J G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol, 2017; 140(3): 645-653. doi: 10.1016/j.jaci.2017.07.004 [7] Yong L, Yu Y, Li B, et al. Calcium/calmodulin-dependent protein kinase IV promotes imiquimod-induced psoriatic inflammation via macrophages and keratinocytes in mice. Nature commun, 2022; 13(1): 4255. doi: 10.1038/s41467-022-31935-8 [8] Juncker M, Kim C, Reed R, et al. ISG15 attenuates post-translational modifications of mitofusins and congression of damaged mitochondriain ataxia telangiectasia cells. Biochim Biophys Acta Mol Basis Dis, 2021; 1867(6): 166102. doi: 10.1016/j.bbadis.2021.166102 [9] Perng Y C, Lenschow D J. ISG15 in antiviral immunity and beyond. Nat Rev Microbiol, 2018; 16(7): 423-439. doi: 10.1038/s41579-018-0020-5 [10] Raposo R A, Gupta R, Abdel-Mohsen M, et al. Antiviral gene expression in psoriasis. J Eur Acad Dermatol Venereol, 2015; 29(10): 1951-1957. doi: 10.1111/jdv.13091 [11] Gao L, Shen J, Ren Y, et al. Discovering novel hub genes and pathways associated with the pathogenesis of psoriasis. Dermatol Ther, 2020; 33(6): e13993. [12] Lu Y, Chen Y, Shi N, et al. L36G is associated with cutaneous antiviral competence in psoriasis. Front Immunol, 2022; 13: 971071. doi: 10.3389/fimmu.2022.971071 [13] Ma J Y, Shao S, Wang G. Antimicrobial peptides: bridging innate and adaptive immunity in the pathogenesis of psoriasis. Chin Med J (Engl), 2020; 133(24): 2966-2975. doi: 10.1097/CM9.0000000000001240 [14] Zhou Y, Wang P, Yan B X, et al. Quantitative proteomic profile of psoriatic epidermis identifies Oas2 as a novel biomarker for disease activity. Front Immunol, 2020; 11: 1432. doi: 10.3389/fimmu.2020.01432 [15] Chen Y, Zhang Z, Zhang Y, et al. Gene set enrichment analysis and ingenuity pathway analysis to verify the impact of Wnt signaling in psoriasis treated with Taodan granules. Am J Transl Res, 2023; 15(1): 422-434. [16] Liu L, Zhou Y, Luo D, et al. Aberrant promoter methylation of Wnt inhibitory factor-1 gene is a potential target for treating psoriasis. Clin Immunol, 2023; 250: 109294. doi: 10.1016/j.clim.2023.109294 -


 投稿系统
投稿系统


 下载:
下载: